Fda 1572 2019-2026


What is the FDA 1572?
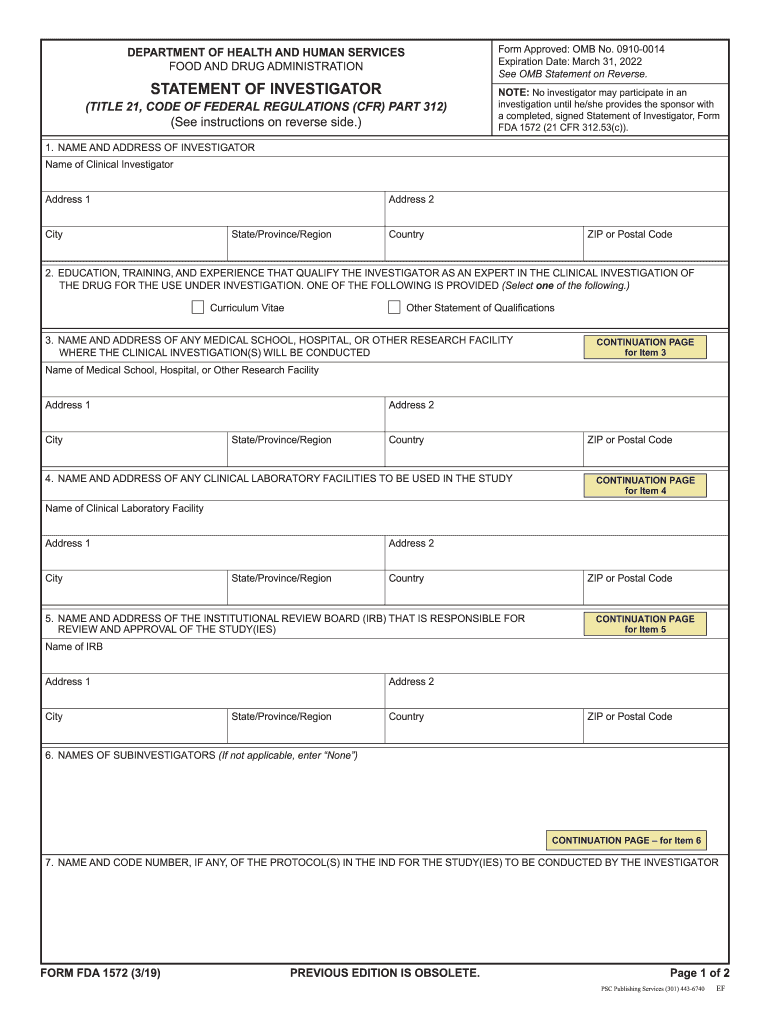
The FDA 1572 form, also known as the Investigator's Statement, is a critical document required by the Food and Drug Administration (FDA) for clinical trials involving investigational drugs. This form serves as a declaration by the investigator, affirming their qualifications and commitment to conducting the trial in compliance with regulatory requirements. It includes essential information such as the investigator's name, address, and qualifications, as well as details about the study site and the study protocol. The FDA 1572 is vital for ensuring that trials adhere to ethical and scientific standards.
Steps to Complete the FDA 1572
Completing the FDA 1572 form involves several key steps to ensure accuracy and compliance. First, gather all necessary information, including your credentials and details about the clinical study. Next, fill out the form by providing your name, contact information, and the names of any sub-investigators. It is also important to include the study title and protocol number. After completing the form, review it carefully for any errors or omissions. Finally, sign and date the form to validate your commitment to the study's compliance with FDA regulations.
Legal Use of the FDA 1572
The legal use of the FDA 1572 form is governed by various regulations that ensure the integrity of clinical trials. This form must be completed accurately and submitted to the FDA as part of the Investigational New Drug (IND) application process. Compliance with the regulations outlined in the form is essential for the legal execution of clinical studies. Failure to adhere to these requirements can result in penalties, including the rejection of the IND application or other legal repercussions. Therefore, understanding the legal implications of the FDA 1572 is crucial for investigators.
Key Elements of the FDA 1572
The FDA 1572 form includes several key elements that are essential for its validity. These elements include the investigator's professional qualifications, the study's title and protocol number, and the location where the study will be conducted. Additionally, the form requires information about any sub-investigators and their roles in the study. The acknowledgment of the investigator's responsibilities, including adherence to Good Clinical Practice (GCP), is also a critical component. Each of these elements plays a vital role in ensuring that the clinical trial is conducted ethically and responsibly.
How to Use the FDA 1572
Using the FDA 1572 form effectively requires understanding its purpose and the context in which it is utilized. The form is primarily used during the initiation of clinical trials to inform the FDA about the investigator's qualifications and the study's details. Once completed, it should be submitted as part of the IND application, along with other required documents. Throughout the study, the investigator must ensure that any changes to the study or personnel are documented and communicated to the FDA, maintaining compliance with regulatory standards.
Form Submission Methods
The FDA 1572 form can be submitted through various methods, depending on the requirements of the specific clinical trial. Typically, the form is submitted electronically as part of the Investigational New Drug (IND) application, which allows for faster processing and easier tracking. In some cases, the form may also be submitted via mail or in person, particularly if there are specific instructions from the FDA or the sponsoring organization. It is important to follow the submission guidelines provided by the FDA to ensure that the form is processed without delays.
Quick guide on how to complete expiration date march 31 2022
Effortlessly Prepare Fda 1572 on Any Device
Digital document management has gained traction among businesses and individuals alike. It serves as an excellent eco-friendly substitute for conventional printed and signed papers, allowing you to obtain the appropriate form and securely save it online. airSlate SignNow provides you with all the tools necessary to create, modify, and electronically sign your documents promptly without delays. Manage Fda 1572 on any platform using airSlate SignNow Android or iOS applications and streamline any document-based task today.
How to Modify and Electronically Sign Fda 1572 with Ease
- Find Fda 1572 and click Get Form to begin.
- Utilize the tools we provide to complete your form.
- Highlight pertinent sections of your documents or obscure sensitive data with tools specifically offered by airSlate SignNow for this purpose.
- Create your signature using the Sign tool, which takes mere seconds and holds the same legal validity as a traditional wet ink signature.
- Review all the information and click on the Done button to save your changes.
- Choose your preferred method to send your form—by email, SMS, invite link, or download it to your computer.
Eliminate the hassle of lost or misplaced files, tedious form navigation, or mistakes that necessitate printing new document copies. airSlate SignNow addresses all your document management needs in just a few clicks from your chosen device. Edit and electronically sign Fda 1572 to ensure seamless communication at any stage of the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Find and fill out the correct expiration date march 31 2022
Create this form in 5 minutes!
How to create an eSignature for the expiration date march 31 2022
How to generate an electronic signature for the Expiration Date March 31 2022 online
How to create an electronic signature for the Expiration Date March 31 2022 in Google Chrome
How to generate an eSignature for putting it on the Expiration Date March 31 2022 in Gmail
How to create an eSignature for the Expiration Date March 31 2022 straight from your mobile device
How to create an electronic signature for the Expiration Date March 31 2022 on iOS
How to create an electronic signature for the Expiration Date March 31 2022 on Android
People also ask
-
What is a 1572 form and why is it important?
The 1572 form, also known as the Statement of Investigator, is crucial for clinical trial compliance. It outlines the qualifications of the investigator, ensuring they are capable of conducting the study. Proper handling of this form is essential to maintain regulatory standards.
-
How can airSlate SignNow help with the 1572 form?
airSlate SignNow simplifies the process of managing the 1572 form by allowing users to easily send and eSign documents electronically. This ensures that the forms are completed quickly and securely, facilitating faster compliance with regulatory requirements.
-
Is there a cost associated with using the airSlate SignNow for the 1572 form?
airSlate SignNow offers various pricing plans designed to accommodate different business needs when handling the 1572 form. Our cost-effective solution ensures you have access to top-notch features without breaking the bank.
-
What features does airSlate SignNow offer for managing the 1572 form?
With airSlate SignNow, users can enjoy features such as document templates, advanced eSignature capabilities, and audit trails specifically for the 1572 form. These features streamline the signing process, ensuring everything is done efficiently and securely.
-
Can airSlate SignNow integrate with other tools to manage the 1572 form?
Yes, airSlate SignNow seamlessly integrates with various applications, making it easier to manage the 1572 form alongside your existing tools. This integration enhances productivity by allowing teams to work within their preferred platforms while ensuring compliance.
-
What are the benefits of using airSlate SignNow for the 1572 form?
Using airSlate SignNow for the 1572 form provides several benefits, including increased efficiency in obtaining signatures, enhanced document security, and improved compliance tracking. These advantages lead to a more organized process for clinical trials.
-
Is airSlate SignNow user-friendly for completing the 1572 form?
Absolutely! airSlate SignNow is designed with user experience in mind, enabling easy navigation for completing the 1572 form. Even those with minimal tech knowledge can effectively use our platform to manage their documents.
Get more for Fda 1572
- Printresetpurchasing agent appointmentform17and de
- 11288b request under section 88b of the inland revenue ordinance cap 112 for a notice of no objection to a company being form
- Personal injury application connecticut judicial branch form
- Initial completion date form
- Ml application form doc
- Adult family home initial licensure checklist f 62671 1100 form
- Fillable online toastmasters form 3 charter membership
- Indiabulls account closure form fill out ampamp sign online
Find out other Fda 1572
- Electronic signature Kansas Plumbing Business Plan Template Secure
- Electronic signature Louisiana Plumbing Purchase Order Template Simple
- Can I Electronic signature Wyoming Legal Limited Power Of Attorney
- How Do I Electronic signature Wyoming Legal POA
- How To Electronic signature Florida Real Estate Contract
- Electronic signature Florida Real Estate NDA Secure
- Can I Electronic signature Florida Real Estate Cease And Desist Letter
- How Can I Electronic signature Hawaii Real Estate LLC Operating Agreement
- Electronic signature Georgia Real Estate Letter Of Intent Myself
- Can I Electronic signature Nevada Plumbing Agreement
- Electronic signature Illinois Real Estate Affidavit Of Heirship Easy
- How To Electronic signature Indiana Real Estate Quitclaim Deed
- Electronic signature North Carolina Plumbing Business Letter Template Easy
- Electronic signature Kansas Real Estate Residential Lease Agreement Simple
- How Can I Electronic signature North Carolina Plumbing Promissory Note Template
- Electronic signature North Dakota Plumbing Emergency Contact Form Mobile
- Electronic signature North Dakota Plumbing Emergency Contact Form Easy
- Electronic signature Rhode Island Plumbing Business Plan Template Later
- Electronic signature Louisiana Real Estate Quitclaim Deed Now
- Electronic signature Louisiana Real Estate Quitclaim Deed Secure