Fda Form 3636 2014


What is the Fda Form 3636
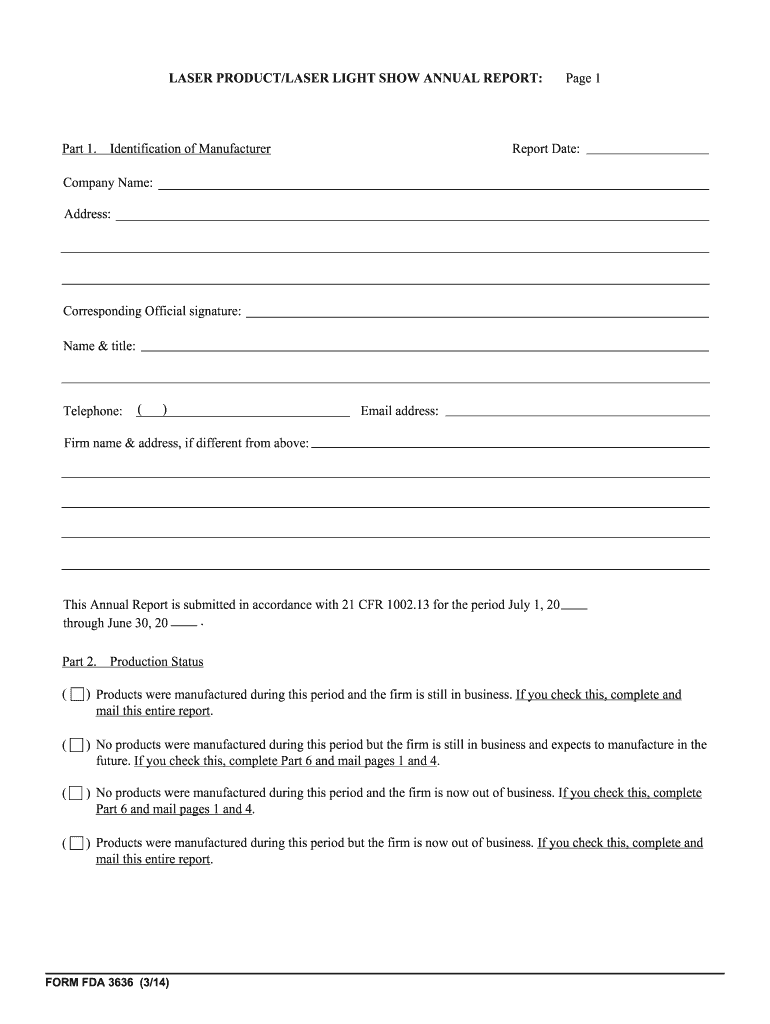
The Fda Form 3636 is a crucial document used in the regulatory process for various applications related to food and drug safety. This form is primarily utilized by businesses and individuals seeking approval or compliance with specific FDA regulations. It serves as a formal request for information or action from the FDA, ensuring that all necessary data is submitted for review. Understanding the purpose and significance of this form is essential for anyone involved in the food and drug industry.
How to use the Fda Form 3636
Using the Fda Form 3636 involves several steps to ensure accurate completion and submission. First, gather all required information, including details about the product or service in question. Next, fill out the form carefully, ensuring that all fields are completed accurately. After completing the form, review it for any errors or omissions. Finally, submit the form according to the instructions provided by the FDA, which may include online submission or mailing the completed document.
Steps to complete the Fda Form 3636
Completing the Fda Form 3636 requires a systematic approach. Begin by downloading the form from the FDA's official website or accessing it through an authorized platform. Follow these steps:
- Read the instructions carefully to understand the requirements.
- Provide accurate information in each section, including contact details and product specifics.
- Attach any necessary supporting documents that may be required.
- Review the completed form for accuracy and completeness.
- Submit the form as directed, ensuring you keep a copy for your records.
Legal use of the Fda Form 3636
The legal use of the Fda Form 3636 is governed by FDA regulations and guidelines. To ensure compliance, it is important to understand the legal implications of submitting this form. The form must be filled out truthfully and accurately, as providing false information can lead to penalties or legal action. Additionally, the form must be submitted within the designated timelines to avoid delays in processing and potential legal ramifications.
Key elements of the Fda Form 3636
Several key elements are essential when completing the Fda Form 3636. These include:
- Applicant Information: Details about the individual or business submitting the form.
- Product Information: Specifics about the product or service related to the submission.
- Purpose of Submission: Clearly stating the reason for the request or application.
- Supporting Documentation: Any additional materials required to substantiate the submission.
Form Submission Methods
The Fda Form 3636 can be submitted through various methods, depending on the specific requirements set by the FDA. Common submission methods include:
- Online Submission: Many applications can be submitted electronically through the FDA's online portal.
- Mail: Completed forms can be sent via postal service to the appropriate FDA office.
- In-Person Submission: Some applicants may choose to deliver the form directly to an FDA facility.
Quick guide on how to complete fda form 3636 2014
Accomplish Fda Form 3636 effortlessly on any device
Digital document management has gained popularity among businesses and individuals. It offers an ideal eco-friendly alternative to conventional printed and signed documents, allowing you to access the correct form and securely save it online. airSlate SignNow provides all the tools necessary to create, modify, and electronically sign your documents promptly without delays. Manage Fda Form 3636 on any platform with airSlate SignNow Android or iOS applications and enhance any document-based workflow today.
How to modify and electronically sign Fda Form 3636 with ease
- Obtain Fda Form 3636 and click Get Form to begin.
- Utilize the tools we offer to fill out your form.
- Emphasize pertinent sections of the documents or redact confidential information using the tools that airSlate SignNow specifically provides for this purpose.
- Create your electronic signature with the Sign tool, which takes mere seconds and holds the same legal validity as a traditional wet ink signature.
- Review the information and click on the Done button to save your changes.
- Choose how you would like to send your form, via email, text message (SMS), or invitation link, or download it to your computer.
Eliminate concerns about lost or misfiled documents, tedious form searching, or mistakes that necessitate reprinting document copies. airSlate SignNow meets your document management needs in just a few clicks from any device of your preference. Edit and electronically sign Fda Form 3636 to ensure excellent communication throughout your form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Find and fill out the correct fda form 3636 2014
Create this form in 5 minutes!
How to create an eSignature for the fda form 3636 2014
How to make an eSignature for a PDF file online
How to make an eSignature for a PDF file in Google Chrome
The way to create an electronic signature for signing PDFs in Gmail
The way to create an eSignature straight from your mobile device
The best way to make an eSignature for a PDF file on iOS
The way to create an eSignature for a PDF document on Android devices
People also ask
-
What is FDA Form 3636 and why is it important?
FDA Form 3636 is a vital document used in the regulatory process for submitting required information to the FDA. It ensures compliance with various federal regulations and facilitates smooth communication between businesses and the FDA. Understanding how to properly complete and submit FDA Form 3636 is crucial for any organization looking to operate within FDA guidelines.
-
How can airSlate SignNow assist with FDA Form 3636 submissions?
airSlate SignNow provides an easy-to-use platform for eSigning and sending FDA Form 3636 documents securely. With our streamlined interface, users can quickly fill out and send the FDA Form 3636, ensuring that submissions are accurate and compliant. This not only saves time but also helps businesses maintain rigorous documentation standards.
-
Is there a cost associated with using airSlate SignNow for FDA Form 3636?
Yes, airSlate SignNow offers various pricing plans tailored to different business needs, including those that frequently handle FDA Form 3636. Our competitive pricing ensures that organizations can efficiently manage their document signing processes without overspending. For specific pricing details, please visit our pricing page.
-
What features does airSlate SignNow offer for managing FDA Form 3636?
airSlate SignNow includes robust features such as customizable templates, bulk sending, and comprehensive tracking for FDA Form 3636. These features simplify the document management process, helping businesses to organize and ensure all necessary forms are completed accurately. Additionally, our cloud-based platform allows for easy access and collaboration on documents.
-
Can I integrate airSlate SignNow with other software to handle FDA Form 3636?
Absolutely! airSlate SignNow seamlessly integrates with various software solutions, making it easy to manage FDA Form 3636 alongside other business systems. Whether you use CRM, project management, or document management tools, our integrations help streamline workflows and improve overall efficiency. Check our integration options for more details.
-
What are the benefits of using airSlate SignNow for FDA Form 3636?
Using airSlate SignNow for FDA Form 3636 signNowly accelerates the submission process while ensuring compliance and reducing errors. Benefits include enhanced document security, improved collaboration, and simplified workflows. By leveraging our solution, businesses can focus more on their core operations and less on administrative tasks.
-
How secure is airSlate SignNow for my FDA Form 3636 documents?
airSlate SignNow prioritizes security with industry-standard encryption and compliance with regulations, ensuring that your FDA Form 3636 documents are protected. We implement rigorous security measures to safeguard data integrity and confidentiality. With airSlate SignNow, you can confidently manage sensitive documents without worrying about security bsignNowes.
Get more for Fda Form 3636
- Samhsa otp mortality report form aatod
- Army hazmat paperwork form
- Industry and urban growth worksheet answers form
- E1 e2 esol literacy reading comprehension punctuation spelling and sentence structure uk adult esol and literacy form
- Affidavit on application to set aside a conviction form
- Application to set aside a conviction an affidavit that is filed in provincial court by a committed person who is applying to form
- Future tokens agreement template form
- Gain share agreement template form
Find out other Fda Form 3636
- How Do I Electronic signature Iowa Construction Document
- How Can I Electronic signature South Carolina Charity PDF
- How Can I Electronic signature Oklahoma Doctors Document
- How Can I Electronic signature Alabama Finance & Tax Accounting Document
- How To Electronic signature Delaware Government Document
- Help Me With Electronic signature Indiana Education PDF
- How To Electronic signature Connecticut Government Document
- How To Electronic signature Georgia Government PDF
- Can I Electronic signature Iowa Education Form
- How To Electronic signature Idaho Government Presentation
- Help Me With Electronic signature Hawaii Finance & Tax Accounting Document
- How Can I Electronic signature Indiana Government PDF
- How Can I Electronic signature Illinois Finance & Tax Accounting PPT
- How To Electronic signature Maine Government Document
- How To Electronic signature Louisiana Education Presentation
- How Can I Electronic signature Massachusetts Government PDF
- How Do I Electronic signature Montana Government Document
- Help Me With Electronic signature Louisiana Finance & Tax Accounting Word
- How To Electronic signature Pennsylvania Government Document
- Can I Electronic signature Texas Government PPT