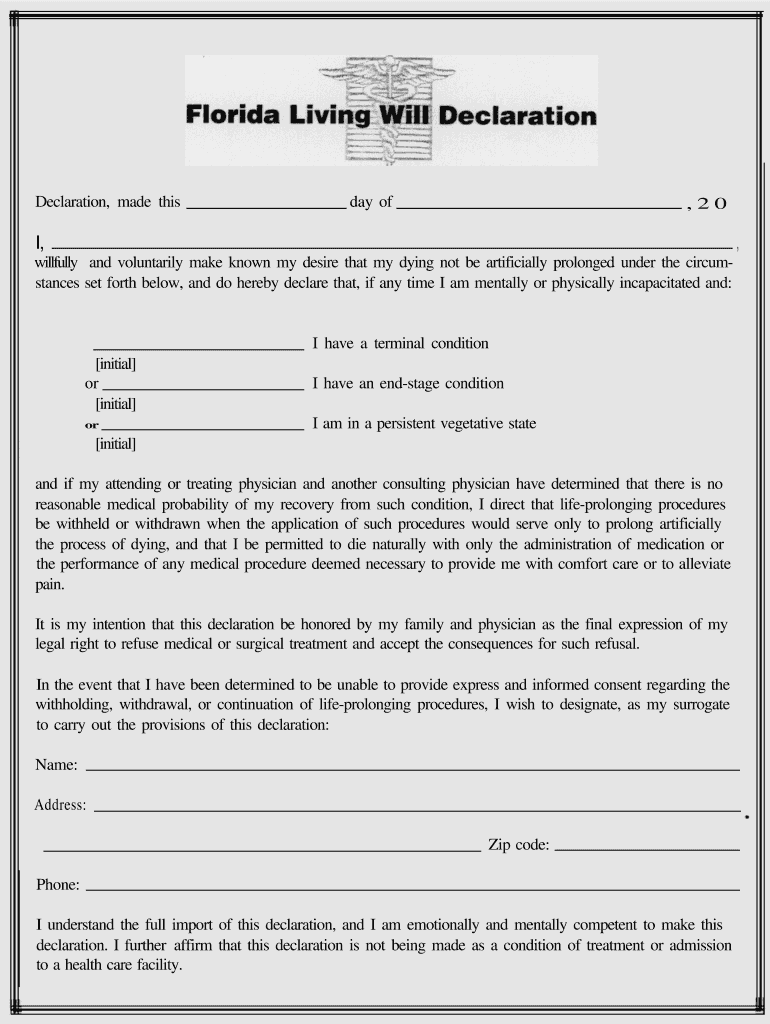
Fill and Sign the Florida Living Will Declaration Form
Helpful suggestions for finalizing your ‘Florida Living Will Declaration Form’ online
Are you fed up with the burden of handling paperwork? Look no further than airSlate SignNow, the premier eSignature solution for individuals and enterprises. Bid farewell to the lengthy process of printing and scanning documents. With airSlate SignNow, you can effortlessly complete and sign documents online. Take advantage of the extensive features included in this user-friendly and cost-effective platform and transform your method of document management. Whether you need to approve forms or gather signatures, airSlate SignNow manages it all seamlessly, with just a few clicks.
Adhere to this detailed guide:
- Sign in to your account or sign up for a complimentary trial with our service.
- Select +Create to upload a file from your device, cloud storage, or our template library.
- Open your ‘Florida Living Will Declaration Form’ in the editor.
- Click Me (Fill Out Now) to prepare the document on your end.
- Add and assign fillable fields for other individuals (if necessary).
- Proceed with the Send Invite options to solicit eSignatures from others.
- Download, print your copy, or convert it into a reusable template.
Don’t be concerned if you need to collaborate with your colleagues on your Florida Living Will Declaration Form or send it for notarization—our platform provides everything required to accomplish such tasks. Register with airSlate SignNow today and elevate your document management to new levels!
FAQs
-
What is a Living Will Declaration and why do I need one?
A Living Will Declaration is a legal document that outlines your preferences for medical treatment in case you become unable to communicate your wishes. It ensures that your healthcare providers follow your directives regarding life-sustaining treatments. Having a Living Will Declaration is essential for protecting your autonomy and ensuring your healthcare decisions are honored.
-
How can airSlate SignNow help me create a Living Will Declaration?
airSlate SignNow provides an easy-to-use platform for drafting and signing your Living Will Declaration. With customizable templates, you can quickly create a document tailored to your needs. Our user-friendly interface makes it simple to fill out, eSign, and share your Living Will Declaration securely.
-
Is there a cost associated with creating a Living Will Declaration using airSlate SignNow?
Yes, while airSlate SignNow offers a free trial, creating a Living Will Declaration typically requires a subscription. Our pricing plans are cost-effective, designed to fit various budgets, and provide access to all features necessary for drafting and managing your Living Will Declaration.
-
Can I store my Living Will Declaration securely with airSlate SignNow?
Absolutely! airSlate SignNow ensures that your Living Will Declaration is stored securely in the cloud. We utilize advanced encryption and security protocols to protect your sensitive documents, giving you peace of mind knowing that your wishes are safeguarded.
-
How does airSlate SignNow facilitate the signing process for my Living Will Declaration?
With airSlate SignNow, you can easily send your Living Will Declaration to designated signers for quick eSigning. Our platform supports multiple signing options, allowing you to obtain signatures from family members or healthcare proxies efficiently. This streamlines the process, ensuring your document is legally binding.
-
Can I update my Living Will Declaration after it's created?
Yes, airSlate SignNow allows you to update your Living Will Declaration at any time. Simply log into your account, access your document, and make the necessary changes. This flexibility ensures that your Living Will Declaration always reflects your current preferences and medical wishes.
-
What features does airSlate SignNow offer for managing my Living Will Declaration?
airSlate SignNow includes various features to help you manage your Living Will Declaration effectively. These features include customizable templates, secure cloud storage, eSigning capabilities, and audit trails to track document activity. All of these tools enhance your experience and ensure your Living Will Declaration is handled professionally.
Find out other florida living will declaration form
- Close deals faster
- Improve productivity
- Delight customers
- Increase revenue
- Save time & money
- Reduce payment cycles

