Fill and Sign the Jubilee Life Insurance Policy Check Online Form
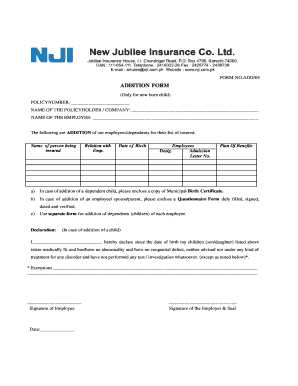
Practical advice on setting up your ‘Jubilee Life Insurance Policy Check Online’ online
Are you fed up with the inconvenience of handling paperwork? Look no further than airSlate SignNow, the leading electronic signature solution for individuals and businesses. Bid farewell to the laborious process of printing and scanning documents. With airSlate SignNow, you can seamlessly finalize and sign paperwork online. Utilize the extensive features bundled into this user-friendly and cost-effective platform and transform your approach to document management. Whether you need to approve forms or gather signatures, airSlate SignNow manages it all effortlessly, with just a few clicks.
Follow this comprehensive guide:
- Log into your account or register for a complimentary trial with our service.
- Click +Create to upload a file from your device, cloud storage, or our template collection.
- Open your ‘Jubilee Life Insurance Policy Check Online’ in the editor.
- Click Me (Fill Out Now) to finish the document on your end.
- Add and assign fillable fields for others (if needed).
- Proceed with the Send Invite options to request eSignatures from others.
- Save, print your copy, or convert it into a reusable template.
Don’t worry if you need to collaborate with others on your Jubilee Life Insurance Policy Check Online or send it for notarization—our solution provides you with everything necessary to accomplish such tasks. Register with airSlate SignNow today and elevate your document management to new levels!
FAQs
-
How can I perform a Jubilee Life Insurance Policy Check Online?
To perform a Jubilee Life Insurance Policy Check Online, visit the official Jubilee Life website and navigate to the policyholder section. You will need your policy number and personal details to access your information securely. This online check provides instant access to your policy status, making it easy to manage your insurance needs.
-
What features does the Jubilee Life Insurance Policy Check Online offer?
The Jubilee Life Insurance Policy Check Online offers a user-friendly interface that allows you to view your policy details, premium status, and coverage information. Additionally, you can download important documents, making it convenient for policy management. This feature ensures you stay informed about your policy without any hassle.
-
Is there a cost associated with the Jubilee Life Insurance Policy Check Online?
No, performing a Jubilee Life Insurance Policy Check Online is completely free for all policyholders. There are no hidden charges or fees associated with accessing your policy information through the online portal. This service is designed to provide transparency and ease of access to your insurance details.
-
What benefits do I gain from using the Jubilee Life Insurance Policy Check Online?
Using the Jubilee Life Insurance Policy Check Online allows you to efficiently manage your policy from the comfort of your home. You can quickly check your policy status, premium payments, and any changes made to your coverage. This accessibility ensures that you are always up-to-date with your insurance needs.
-
Can I update my personal information through the Jubilee Life Insurance Policy Check Online?
Yes, you can update your personal information through the Jubilee Life Insurance Policy Check Online. After logging in, navigate to the profile section to make necessary changes. Keeping your information up-to-date is crucial for effective communication regarding your policy.
-
What security measures are in place for the Jubilee Life Insurance Policy Check Online?
The Jubilee Life Insurance Policy Check Online employs advanced encryption technology to ensure your data is secure. All personal and policy information is protected against unauthorized access, giving you peace of mind while managing your insurance online. Your privacy and security are a top priority.
-
How do I access customer support for issues related to the Jubilee Life Insurance Policy Check Online?
If you encounter any issues with the Jubilee Life Insurance Policy Check Online, you can contact customer support through the live chat feature on the website or by calling their dedicated helpline. They offer prompt assistance to resolve any concerns you may have regarding your policy. Support is available during business hours for your convenience.
Find out other jubilee life insurance policy check online form
- Close deals faster
- Improve productivity
- Delight customers
- Increase revenue
- Save time & money
- Reduce payment cycles

