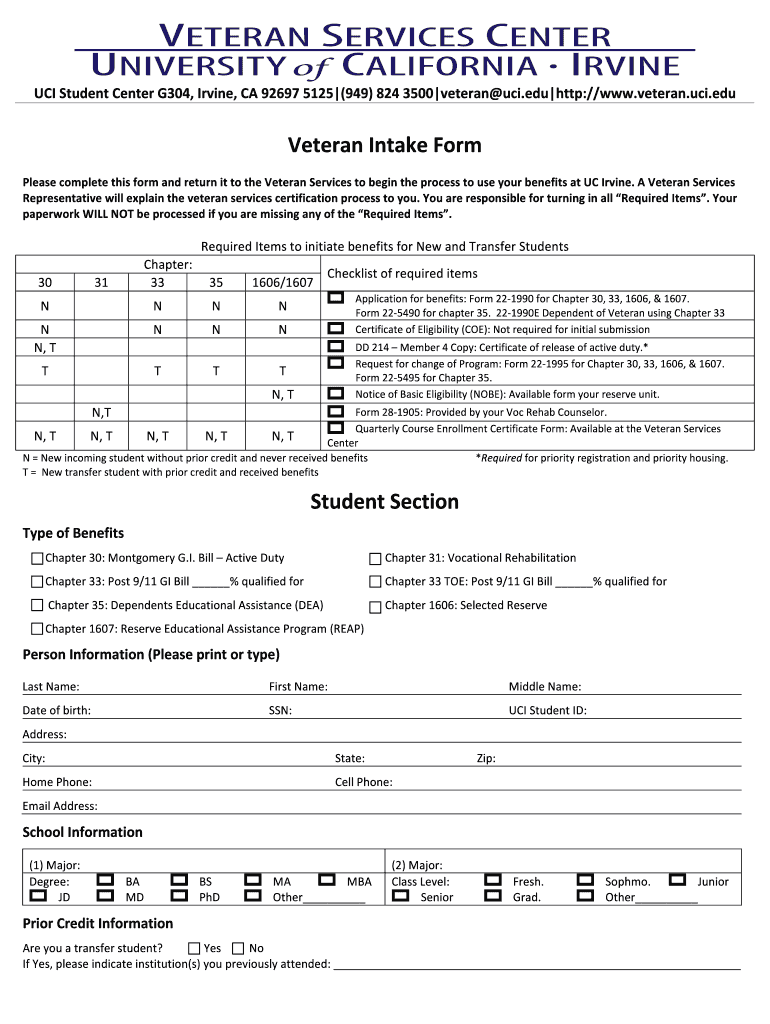
Fill and Sign the Intake Form2 Veteran Uci
Useful advice for finalizing your ‘Intake Form2 Veteran Uci’ online
Are you fed up with the inconvenience of managing paper documents? Look no further than airSlate SignNow, the premier e-signature solution for individuals and organizations. Bid farewell to the monotonous routine of printing and scanning documents. With airSlate SignNow, you can effortlessly finalize and sign documents online. Utilize the extensive features embedded in this user-friendly and cost-effective platform and transform your method of document management. Whether you need to sign forms or gather signatures, airSlate SignNow takes care of it all with ease, needing just a few clicks.
Follow this comprehensive guide:
- Access your account or sign up for a complimentary trial of our service.
- Click +Create to upload a document from your device, cloud storage, or our template library.
- Edit your ‘Intake Form2 Veteran Uci’ in the editor.
- Select Me (Fill Out Now) to set up the document on your end.
- Add and designate fillable fields for other users (if needed).
- Proceed with the Send Invite options to solicit eSignatures from others.
- Download, print your copy, or convert it into a reusable template.
Don’t fret if you need to collaborate with your colleagues on your Intake Form2 Veteran Uci or send it for notarization—our platform has everything necessary to accomplish these tasks. Join airSlate SignNow today and elevate your document management to a new height!
FAQs
-
What is the Intake Form2 Veteran Uci offered by airSlate SignNow?
The Intake Form2 Veteran Uci is a specialized document designed for veterans to streamline their intake process. By utilizing airSlate SignNow, organizations can create, send, and eSign this form efficiently, ensuring that veterans receive the care they need without unnecessary delays.
-
How does the Intake Form2 Veteran Uci improve the document signing process?
The Intake Form2 Veteran Uci simplifies the document signing process by allowing users to eSign electronically from anywhere, at any time. This not only saves time but also enhances accuracy, as it reduces the chances of errors associated with traditional paper forms.
-
What are the pricing options for using airSlate SignNow with the Intake Form2 Veteran Uci?
airSlate SignNow offers flexible pricing plans tailored to different business needs, including options for individuals, small businesses, and enterprises. By choosing a plan that fits your organization, you can effectively manage the Intake Form2 Veteran Uci without breaking the bank.
-
Can I customize the Intake Form2 Veteran Uci to fit my organization’s needs?
Absolutely! The Intake Form2 Veteran Uci can be fully customized using airSlate SignNow's easy-to-use platform. You can modify fields, add branding, and incorporate any specific questions or requirements to ensure it meets your organization's unique needs.
-
What integrations does airSlate SignNow offer for the Intake Form2 Veteran Uci?
airSlate SignNow seamlessly integrates with various applications such as Google Drive, Dropbox, and CRM systems. This allows you to manage and store your Intake Form2 Veteran Uci alongside your existing tools, enhancing workflow efficiency.
-
What security features does airSlate SignNow provide for the Intake Form2 Veteran Uci?
Security is a top priority for airSlate SignNow, especially when handling sensitive documents like the Intake Form2 Veteran Uci. The platform employs advanced encryption, secure data storage, and compliance with industry regulations to protect your information.
-
How can the Intake Form2 Veteran Uci benefit veterans and organizations alike?
The Intake Form2 Veteran Uci benefits veterans by providing a faster, more efficient way to submit necessary information, ensuring they receive timely assistance. Organizations also benefit by streamlining their intake processes, reducing paperwork, and enhancing overall service delivery.
Find out other intake form2 veteran uci
- Close deals faster
- Improve productivity
- Delight customers
- Increase revenue
- Save time & money
- Reduce payment cycles

