Universal Data Collection
CDC ID
U.S. Department of Health
and Human Services
Annual Visit
Public Health Service
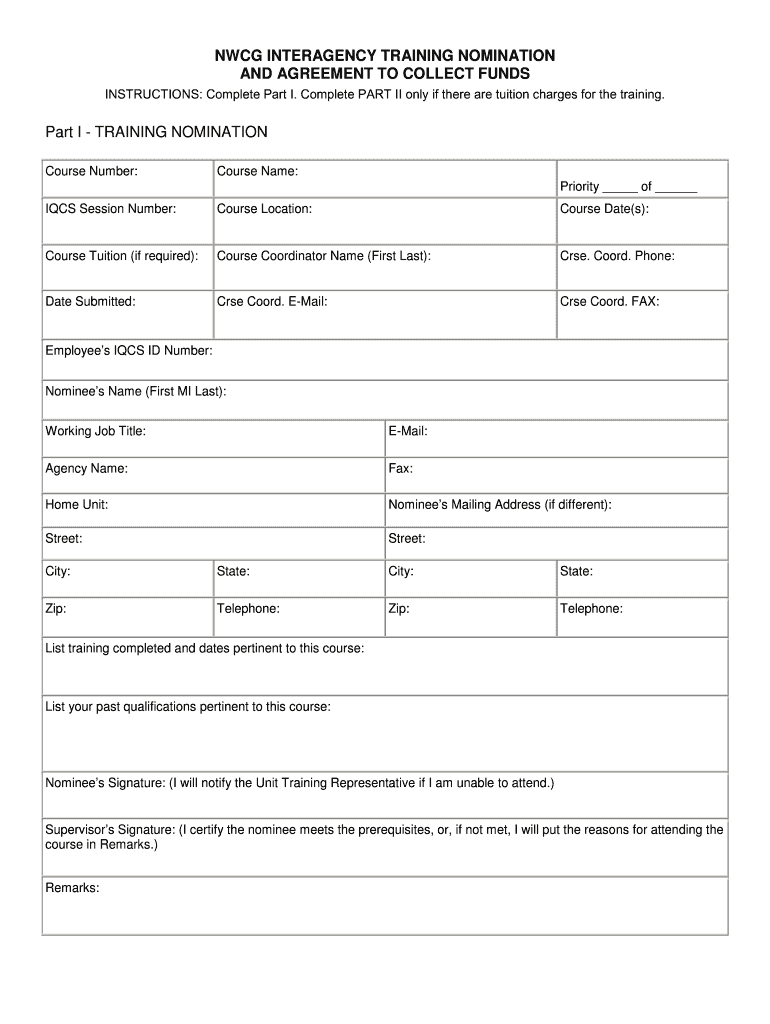
GENERAL INFORMATION
Date of visit
Date form completed
Month
Day
Year
Month
Form completed by
Day
Year
Data entered by
CDC Use Only
DEMOGRAPHIC INFORMATION
1. Zipcode:
(first 3 digits)
5. Employment status (check one):
7. Health Insurance
(check all that apply):
Straight commercial insurance
Employed full-time
2. Weight:
kg
.
3. Height:
Employed part-time
Commercial insurance HMO
Not employed
cm
4. Education: Check the highest
education level completed by patient
Pre-elementary
Commercial insurance PPO
If not employed, check one of the
following:
Child or student
Straight Medicare
Medicare HMO
Straight Medicaid
Homemaker
Medicaid HMO
Able, but not currently working
Primary / Secondary
TRICARE
Permanently disabled
State high-risk insurance plan
Enter grade 1-12
Retired
Uninsured
Technical school
Other ________________________
Other ________________________
College degree
6. HTC utilization (check one):
Advanced degree
8. Has the patient had an
analysis of his or her genetic
mutation since birth or the last
visit?
Frequent (visits HTC once per year)
Other ______________________
4a. Current student:
Yes
No
Infrequent (visits HTC every 2-3 years)
Rare (visits HTC every 4 or more years)
Yes
First visit
No
TREATMENT INFORMATION
9. Treatment type (check one):
12. Bleeding episodes in the last 6 MONTHS (If none, enter zero):
Episodic care
Based on infusion logs
Immune tolerance
OR
Number of joint bleeds
Continuous
OR
Number of other bleeds
If prophylaxis,
OR
Number of muscle bleeds
Prophylaxis
Estimated by patient recall
OR
Intermittent
10. Highest inhibitor titer since
and including the last visit:
(Bethesda units):
13. Intracranial hemorrhage (ICH) since last annual visit?
13a. If yes, date
Month
not done
13b. If yes, associated with
.
11. Immune tolerance therapy
since the last annual visit:
Yes
No
Unknown
Day
Year
Trauma
Thrombocytopenia
Other _________________
14. Home infusion?
Yes
No
14a. If yes, infused by (check all that apply)
If yes,
Patient
Successful
Family member
Unsuccessful
Medical care provider
CDC 59.8C 10/2005
(Page 1 of 8)
Annual Visit Form
Yes
No
�ANNUAL VISIT FORM
FORM COMPLETION: Complete this form for all consenting, eligible patients attending the treatment center. If this is the first visit for which this form is
being completed, substitute “during the previous 12 months” for the phrase “since the last annual visit.”
Patient CDC ID: The unique 12-digit number generated for each patient by staff at the hemophilia treatment center (HTC) using the CDC ID computer
program.
General Information: Enter the date of the visit and the date that this form was completed. Enter the initials of the person completing the form.
DEMOGRAPHIC INFORMATION
1. Zipcode of Residence: Enter the first three digits of the zipcode for the patient’s residence (NOT for the HTC that the patient visits).
2. Weight & 3. Height: Enter the patient’s weight in kilograms and height in centimeters without shoes and in light clothing; (2.2 lbs = 1 kg)
(1 inch = 2.54cm). To convert pounds to kilograms divide by 2.2. To convert inches to centimeters multiply by 2.54.
4. Education: As of the date of the visit, check PRIMARY / SECONDARY and enter the highest completed grade level for grades 1-12 or check appropri
ate box for education beyond high school. Check PRE-ELEMENTARY if the participant has not begun elementary school or has not completed the first
grade.
4a. Current Student: As of the date of the visit, check YES if the patient is a full-time or part-time student. Otherwise, check NO.
5. Employment status: As of the date of the visit, check the employment status of the patient. If EMPLOYED, check either FULL-TIME or PART-TIME.
If NOT EMPLOYED, check the most appropriate reason for unemployment. In order to check PERMANENTLY DISABLED, the patient must have quali
fied for disability income and must not work at all. In order to check RETIRED, the patient must be of retirement age (usually >55 years) and not working
at all.
6. HTC utilization: Use the history of patient visits to the HTC to determine whether the patient utilizes the HTC on a frequent, infrequent, or rare basis.
Count only actual visits to the HTC, not phone contacts or written correspondence. Check FIRST VISIT if the current visit is the first visit to the HTC.
7. Health insurance: Check all sources of health insurance coverage for the patient. Please see Data Forms Manual for more detail about health insur
ance plans.
Commercial Insurance: Insurance provided through private or public companies and paid for either by employers or by individuals.
Medicare: Federal health insurance program available to persons who are either over 65 years old or disabled.
Medicaid (Medical Assistance, Title 19): State/federal health insurance program for certain needy and low income individuals.
TRICARE: Insurance offered by the federal government to persons who are in the military and their dependents.
State High Risk Insurance Plan: State-sponsored insurance plan for individuals who have difficulty purchasing insurance due to a
pre-existing condition.
Uninsured: Persons without any health insurance coverage.
Other: Any other type of health insurance plan not listed above.
8. Has the patient had an analysis of his or her genetic mutation: Check YES, if the patient has undergone genetic testing to determine the specific
genetic mutation responsible for his/her bleeding disorder. If they have not undergone testing or if it is unknown, check NO.
TREATMENT INFORMATION
9. Treatment type (check one): If the patient received treatment products only in response to bleeding complications since the last annual visit, check
EPISODIC CARE. If the patient is currently undergoing immune tolerance therapy (either initial or maintenance), check IMMUNE TOLERANCE. If the
patient received treatment products to prevent bleeding or to prevent rebleeding, check PROPHYLAXIS. If you check PROPHYLAXIS and the patient
was recommended to receive treatment products on a regular schedule to prevent any and all bleeding and this therapy was expected to continue indefi
nitely, check CONTINUOUS. If you check PROPHYLAXIS and the patient received treatment products on a regular schedule for a period of at least 28
days on at least one occasion since the last annual visit and this therapy was not expected to continue for an indefinite period of time, check INTERMIT
TENT. You should not check prophylaxis if the patient received treatment products for less than a period of 28 days.
10. Highest inhibitor titer since and including the last visit: Enter the highest inhibitor titer measured at or since the last annual visit. Do not include
values measured at the current annual visit. If less than 1, enter 0 (zero) in the box before the decimal point when entering the test result.
11. Immune tolerance therapy since the last annual visit: If the patient received immune tolerance therapy since the last annual visit, check YES. If
yes, also check whether the therapy was SUCCESSFUL or UNSUCCESSFUL . Check SUCCESSFUL only if the patient can be effectively treated for a
bleeding episode with a factor dosage appropriate to his/her hemophilia severity and its type; otherwise, check UNSUCCESSFUL. Check NO if the
patient did not receive immune tolerance therapy since the last annual visit. Check UNKNOWN if it is not known whether or not the patient received
immune tolerance therapy since the last annual visit.
12. Bleeding into a joint, muscle, or other area: Enter the number of times in the last 6 MONTHS that the patient has had a bleed into a joint, muscle,
or other area. Use infusion logs provided by the patient, if available. Otherwise, estimate as best as possible, the number of bleeds based on interview
with the patient. Include bleeds which occurred secondary to medical procedures. If patient experienced no bleeds at one or more of the sites, enter 0
(zero) for that/those site(s).
13. Intracranial hemorrhage: Check YES, if the patient has received a diagnosis of an intracranial hemorrhage (ICH) by a physician since the last annu
al visit.
13a. If yes, indicate the date of first diagnosis
13b. Check whether the bleed was associated with TRAUMA, THROMBOCYTOPENIA, or some OTHER complication.
14. Home infusion: Check YES, if the patient receives treatment products intravenously outside of the medical setting.
14a. If YES, check whether the product is infused by the patient, a family member, or a medical care provider. If the patient receives treatment prod
ucts only in a medical setting (e.g., HTC, emergency room), check NO.
CDC 59.8C 10/2005
(Page 2 of 8)
Annual Visit Form
�CDC ID
TREATMENT PRODUCTS
15. Treatment product(s) used since the last annual visit: (check all that apply)
NONE USED
UNKNOWN
Factor VIII, vWF, or Non-plasma Products
Factor IX, PCC, and Other Factor Products
Recombinant FIX
BeneFIX
Recombinant FVIII
Advate
Helixate FS
Other, specify ______________________
Kogenate FS
Recombinate
Human FIX
AlphaNine S-D
ReFacto
Other, specify ______________________
Mononine
Other, specify ______________________
Monoclonal FVIII
Hemofil M
Prothrombin complex
Bebulin VH
Monarc-M
Monoclate P
Profilnine SD
Other, specify ______________________
Proplex T
Other, specify ______________________
Human FVIII containing VWF
Alphanate
Humate P
Activated prothrombin complex
Autoplex T
Koate DVI
FEIBA VH
Other, specify ______________________
Other, specify ______________________
Porcine factor VIII
Other, specify ______________________
Concentrates of other factors
NovoSeven (FVIIa)
Fibrogammin P (FXIII)
Blood bank products
Cryoprecipitate
Other, specify ______________________
Fresh-frozen plasma
Platelets
Packed RBCs or whole blood
Non-plasma and topical products
Intravenous desmopressin (DDAVP)
Nasal desmopressin (Stimate)
Amicar
Fibrin glue
Other, specify ______________________
CDC 59.8C 10/2005
(Page 3 of 8)
Annual Visit Form
�ANNUAL VISIT FORM
15. Treatment product(s) used since the last annual visit: Check or enter all treatment products (including blood products, DDAVP, or Amicar) used
by the patient since the last annual visit. Check NONE USED, if the patient did not use any treatment products since the last annual visit. Check
UNKNOWN, if you do not know whether the patient used treatment products since the last annual visit or if you are unable to determine which products
were used.
CDC 59.8C 10/2005
(Page 4 of 8)
Annual Visit Form
�CDC ID
INFECTIOUS DISEASE
16. Risk factors for liver disease:
16a. Past/present hepatitis infection
Positive HBsAg and/or anti-HBC and/or
anti-HBS in absence of vaccination.
Yes
No
Unknown/untested
Yes
No
20a. If yes, agent used (see reverse for brands):
16b. Other risk factors (check all that apply)
Interferon
History of alcohol abuse
Other_____________
Lamivudine
20c. Hepatitis C genotype:
Yes
1a or 1b
Pegylated Interferon
Other ____________________
No
Unknown
other than 1a or 1b
Unknown
21. Has the patient used a CVAD since the last annual visit?
Yes
(check all that apply)
Ascites
Ribavirin
20b. If yes, sustained response?
None
17. Signs or symptoms of liver
disease since the last annual visit:
Jaundice
Not measured
20. Has the patient received any therapy for chronic viral
hepatitis?
Yes
No
Unknown/untested
Other ______________
No
19. Has the patient had an elevated prothrombin time (PT) since
the last annual visit?
Yes
No
Not measured
Positive anti-HCV and/or RIBA and/or PCR
Yes
18. Does the patient have chronically elevated ALT/AST levels?
No
21a. If yes, type of CVAD (check all that apply)
None
Port
21b. If yes, any infection in CVAD since last visit?
Varices
Yes
Catheter
PICC
No
RISK REDUCTION
22. What is the HIV status of the patient?
26. How often is a condom used when having sex?
Positive
if positive and age ≥16, go to item 23.
Does not have sex (practices abstinence)
Negative
if negative, skip to item 29.
Never
Untested
if untested, skip to item 29.
Less than 50% of occasions
23. Does the patient have a regular partner?
Yes
No
Usually (50–89% of occasions)
Nearly always (90–99% of occasions)
Always
24. If yes, has this patient’s regular partner ever
Yes
No
Unknown
been tested for HIV?
24a. If yes, was the result positive?
Yes
No
Unknown
25. How many pregnancies in patient or
among any sex partners impregnated by
this patient since the last annual visit?
27. How many sex partners of this patient were
tested for HIV since the last annual visit? _____
28. How many sex partners of this patient have
tested newly positive for HIV since the last
annual visit? _____
JOINT DISEASE
29. How often since the last annual visit has the
patient used a cane, crutches, or walker for
ambulation or mobility?
Never
Intermittently
Always
30. How often since the last annual visit has the
patient used a wheelchair for mobility?
Never
Intermittently
Always
31. How many days since the last annual visit has
the patient missed work or school because of
lower extremity joint problems?
days
not applicable
32. How many days since the last annual visit
has the patient missed work or school because
of upper extremity joint problems?
days
CDC 59.8C 10/2005
not applicable
(Page 5 of 8)
33. Has the patient experienced a joint
infection since the last annual visit?
Yes
No
34. Check the statement which best describes
the patient’s current overall activity level:
Unrestricted school/work and recreational activities
Full school/work with limited recreational activity levels
due to pain, loss of motion, weakness
Limited school/work and recreational activity levels
due to pain, loss of motion, weakness
Limited school/work, recreational activity levels, and selfcare activity levels due to pain, loss of motion, weakness
Requires assistance from another person for school/work/
self-care, and unable to participate in recreation due to
pain, loss of motion, weakness
Annual Visit Form
�ANNUAL VISIT FORM
INFECTIOUS DISEASE INFORMATION
16. Risk factors for liver disease:
16a. Past or present infection with hepatitis B or C based on previous laboratory testing (not clinical signs or symptoms). Laboratory test results
that indicate past or present infection with HBV include a positive HBsAg and/or anti-HBC, or anti-HBS in the absence of vaccination. Test
results that indicate past or present infection with HCV include a positive anti-HCV and/or RIBA, and/or PCR for HCVRNA.
16b. Check or enter any other risk factors for the development of liver disease such as a history of alcohol abuse, or other exposures. Check
NONE, only if the patient has no known risk factors for liver disease.
17. Signs or symptoms of liver disease: Check or enter any signs or symptoms of liver disease experienced by the patient since the last annual
visit. Check NONE, if no signs or symptoms were present.
18. Chronically elevated liver enzymes: Check YES if ALT levels measured in the past were at least 1.5 times the upper limit of normal on at least
two occasions separated by at least one month and the most recent measurement was within one year of this visit. Do not include measurements
made at the current annual visit.
19. Elevated PT: Check YES, if a measured prothrombin time (PT) was greater than 1.5 times the upper limit of normal at or any time since the last
annual visit.
20. Any therapy for chronic viral hepatitis: Check YES, if the patient has received antiviral therapy for chronic viral hepatitis ever in the past if
this is the first time the form is being completed or since the last annual visit if the form is being completed in subsequent years.
20a. If Yes, check whether therapy included pegylated interferon (PEG-Intron, Pegasys), interferon (Intron A, Roferon A), ribavirin (Rebetrol,
Copegus, Ribasphere), lamivudine (3TC, Epivir) and/or some other agent (please stipulate which other agent). Check all that apply.
20b. If Yes, check whether or not treatment resulted in a sustained response. A SUSTAINED RESPONSE is defined as normalization of the
ALT level for at least 6 months after the end of therapy AND either seroconversion from HBeAg positive to negative (if treated for chronic
hepatitis B infection) or from HCV RNA positive to negative by PCR (if treated for chronic hepatitis C infection).
20c. Please check the Hepatitis C genotype. Check unknown if the genotype is not known.
21. Use of Central Venous Access Devices (CVAD): Check YES, if the patient used any type of CVAD at any time since the last annual visit.
Otherwise, check NO.
21a. If yes, check all types of CVAD used since the last visit. PORT is an internal CVAD that is placed surgically under the skin of the chest or
arm and is accessed using a needle. CATHETER can be either tunneled or non-tunneled, permanent or temporary and is inserted directly
into a central vein such as the subclavian, jugular, or femoral (e.g., Hickman, Broviac). PERIPHERALLY INSERTED CENTRAL CATHETER
(PICC) is inserted into a peripheral vein in the upper arm or leg and threaded into a central vein such as the subclavian or jugular vein.
21b. If yes, also check whether or not the patient was diagnosed with an CVAD associated infection by a physician since the last annual visit.
RISK REDUCTION INFORMATION
22. HIV status of the patient: Check POSITIVE, if the patient has ever tested HIV antibody positive. Check NEGATIVE if the patient has tested HIV
antibody negative. Check UNTESTED, if the patient has not been tested for antibodies to HIV or if the results of testing are not known.
23. Regular partner: Ask the patient if there is a person whom he/she has dated, lived with, or been married to for at least three (3) months.
24. HIV testing of patient’s regular partner: Complete this item only if the patient has a regular partner. Check YES, if the patient’s regular partner
has ever been tested for HIV. If not, check NO. If it is not known whether the regular partner has been tested, check UNKNOWN.
24a. If the regular partner has been tested, check YES if the test result was positive; check NO if the test result was negative.
If the test result is not known, check UNKNOWN.
25. Number of pregnancies in patient or among any sex partners impregnated by this patient since the last annual visit:
Enter the number of pregnancies occuring in this patient or in those sex partners impregnated by this patient since the last annual visit. If none are
known, enter 0 (zero).
26. Condom use: Ask the patient how often a condom is used when having sex (includes anal, vaginal, or oral). Check the category that most
accurately reflects condom use.
27. Number of patient’s sex partners tested for HIV since the last annual visit: Enter the number of sex partners of this patient who are known
to have had HIV testing since the last annual visit. If none are known or none have been tested, enter 0 (zero).
28. Number of patient’s sex partners testing newly positive for HIV since the last annual visit: Enter the number of sex partners of this patient
who are known to have had a positive test for HIV for the first time since the last annual visit. If none are known or none have been tested, enter 0
(zero).
JOINT DISEASE INFORMATION
29 & 30. Use of cane/crutches or walker and use of wheelchair or equivalent since the last annual visit: If the patient never used the above,
check NEVER. If the patient used one or more of the above but only with an acute bleeding episode, check INTERMITTENTLY. If the patient used
one or more of the above with usual ambulation since the last visit, check ALWAYS.
31 & 32. Work or school missed because of a lower or an upper extremity joint problem since last annual visit: Enter the number of days
since the last annual visit that the patient reports missing work or school because of a complication (e.g., bleed, infection, arthritis, pain) in a lower
(#31) or an upper (#32) extremity. If the patient does not attend work or school, check NOT APPLICABLE.
33. Joint infection since the last visit: Check YES, if the patient experienced a physician-diagnosed infection in a joint since the last annual visit;
otherwise, check NO.
34. Overall activity level: Check the statement that BEST describes overall current activity level as impacted by joint disease according to the
patient.
CDC 59.8C 10/2005
(Page 6 of 8)
Annual Visit Form
�Universal Data Collection
CDC ID
U.S. Department of Health
and Human Services
Public Health Service
35. Ranges of motion
CODES*
Date ROM measurements performed
Month
Day
Orthopedic
appliance
Not measured
A = Acute bleed
B = Post-op restrictions
C = Other medical reason
Year
Invasive procedure
D = Cast
E = Splint
F = Orthosis
G = Brace
H = Arthrodesis
I = Joint replacement
J = Arthroscopic synovectomy
K = Open synovectomy
L = Radioisotopic synovectomy
M = Other invasive procedure
*See reverse for definitions
Record ROM Endpoint
Left
Joint and Measuring Position
Check
Target
joint
Right
Hip
Circle all codes that apply from list above
Orthopedic
Appliance
Not measured
Hip
Invasive procedure
Hip
Extension (sidelying)
L:
L:
A B C
L:
D E F G
L:
H I J K L M
Flexion (supine)
R:
R:
A B C
R:
D E F G
R:
H I J K L M
Knee
Knee
Knee
Flexion (supine)
L:
L:
A B C
L:
D E F G
L:
H I J K L M
Extension (supine)
R:
R:
A B C
R:
D E F G
R:
H I J K L M
Hyperextension (supine)
Shoulder
Shoulder
Flexion (supine)
Shoulder
L:
L:
A B C
L:
D E F G
L:
H I J K L M
R:
R:
A B C
R:
D E F G
R:
H I J K L M
Elbow
Flexion (supine)
Extension (supine)
Elbow
Elbow
L:
L:
A B C
L:
D E F G
L:
H I J K L M
R:
R:
A B C
R:
D E F G
R:
H I J K L M
Hyperextension (supine)
Pronation (sitting)
Supination (sitting)
Ankle
Dorsiflexion (sitting)
Plantarflexion (sitting)
Ankle
L:
L:
A B C
L:
D E F G
L:
H I J K L M
R:
R:
A B C
R:
D E F G
R:
H I J K L M
Ranges of motion measured by (check one):
CDC 59.8C 10/2005
(Page 7 of 8)
Ankle
Physical therapist
Other
Annual Visit Form
�ANNUAL VISIT FORM
35. Ranges of Motion:
Date Measured -
Enter the date on which the ROM measurements were performed.
ROM Endpoint -
Measure the ROM to the nearest degree using the techniques described in the UDC Joint
Range of Motion Reference Guide. All measurements should be obtained by passively moving
each joint to its endpoint. Record the ROM measurement endpoint for each joint under the col
umn labeled LEFT or RIGHT as appropriate. If there is hyperextension in a joint, record the num
ber of degrees of hyperextension as a positive number and place a zero in the extension box.
The hip and knee extensions, ankle and elbow measurements may be a negative number (see
the UDC Joint Range of Motion Reference Guide pp. 4-5 for details). Be sure to include the
minus sign for a negative number.
Target Joint -
If the joint being measured is currently designated as a target joint (defined as recurrent bleeding
into the joint on four (4) or more occasions in the past 6 months), check the box in the target
joint column under the appropriate joint. L = left and R = right.
Not Measured -
If the joint ROM cannot be measured during this visit, circle the letter code(s) for the reason(s)
that the joint ROM could not be measured. The codes for not measured are:
A = Acute Bleed (Bleed which occurs within 24 hours of the visit)
B = Post-operative Restrictions (Movement restrictions due to a procedure or recent surgery)
C = Other Medical Reason (Any other medical reason)
Orthopedic
Appliance -
If the patient has been prescribed or has used an orthopedic appliance on the joint being meas
ured since the last annual visit, circle the letter code in the orthopedic appliance column under
the appropriate joint. L= Left and R= Right. If more than one appliance was used since the last
visit, circle all that apply. The codes for orthopedic appliance are:
D = Cast (a solid rigid dressing made of plaster or fiberglass that is molded to a body part)
E = Splint (an appliance often made of moldable plastic used for temporary support of a joint)
F = Orthosis (an appliance often made of durable plastic by an orthotist for long-term joint sup
port)
G = Brace (an appliance often made of fabric or neoprene, including ace wraps, used for joint
support during a sporting or work activity)
Invasive Procedure - If the patient has undergone an invasive procedure in the joint being measured since the last
annual visit, circle the letter code in the invasive procedure column under the appropriate joint.
L= Left and R= Right. If more than one invasive procedure was performed since the last visit, cir
cle all that apply. The codes for invasive procedure are:
H = Arthrodesis (Artificial ankylosis, fixation, or fusion)
I = Joint Replacement (Artificial joint insertion)
J = Arthroscopic synovectomy (Removal of synovium by endoscope)
K = Open synovectomy (Removal of synovium using a surgical procedure to open the joint)
L = Radioisotopic synovectomy (Removal of synovium by injection of radioisotopes)
M = Other Invasive Procedure (Any other invasive procedure)
Measurements
Made By -
CDC 59.8C 10/2005
Indicate by checking the appropriate box whether the ROM measurements were made by a
physical therapist or by some other health care professional (e.g., nurse, physician, etc.).
(Page 8 of 8)
Annual Visit Form
�