Sign Clinical Trial Agreement
- Quick to start
- Easy-to-use
- 24/7 support
Simplified document journeys for small teams and individuals



We spread the word about digital transformation
Why choose airSlate SignNow
-
Free 7-day trial. Choose the plan you need and try it risk-free.
-
Honest pricing for full-featured plans. airSlate SignNow offers subscription plans with no overages or hidden fees at renewal.
-
Enterprise-grade security. airSlate SignNow helps you comply with global security standards.







Superior form management with airSlate SignNow
Gain access to a rich form collection
Create reusable templates
Collect signatures through links
Keep forms safe
Improve collaboration
eSign via API integrations
Your complete how-to guide - clinical trial agreement
At present, you probably won't find a company that doesn't use modern day technology to atomize workflow. A digital signing is no longer the future, but the present. Modern day companies using their turnover simply cannot afford to give up on-line software that provide advanced data file processing automation tools, such as Sign Clinical Trial Agreement function.
How you can deal with Sign Clinical Trial Agreement airSlate SignNow feature:
-
When you get to our website, Login or make your account if you don't have one, it will take you a couple of seconds.
-
Upload the appropriate data file or choose one from your catalogue folders: Documents, Archive, Templates.
-
As a result of cloud-based storage compatibility, you can quickly upload the needed doc from favored clouds with practically any gadget.
-
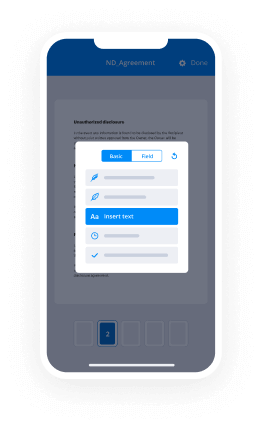
You'll get your data file opened within the up-to-date PDF Editor where you can make changes before you carry on.
-
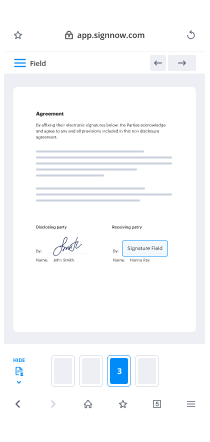
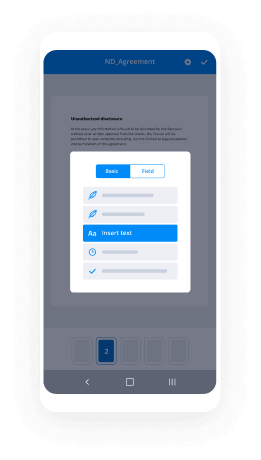
Type textual content, insert pictures, add annotations or fillable fields to be completed further.
-
Use My Signature button for self-signing or place Signature Fields to deliver the eSign request to a single or several users.
-
Use the DONE button when completed to carry on with Sign Clinical Trial Agreement feature.
airSlate SignNow browser solution is important to boost the efficiency and performance of all working processes. Sign Clinical Trial Agreement is among the capabilities that will help. Making use of the internet-based application nowadays is actually a necessity, not just a competing benefit. Try it now!
How it works
Rate your experience
What is the clinical agreement template
The clinical agreement template is a formal document used to outline the terms and conditions of a clinical trial. It serves as a binding contract between parties involved, such as researchers, sponsors, and institutions. This template typically includes details about the study's objectives, responsibilities of each party, confidentiality agreements, and compliance with regulatory standards. By using this template, organizations can standardize their agreements, ensuring all necessary legal and ethical considerations are addressed.
How to use the clinical agreement template
Using the clinical agreement template involves several steps to ensure it meets the specific needs of your clinical trial. Start by reviewing the template thoroughly to understand its structure and required information. Customize the sections to reflect the particulars of your trial, including participant roles, timelines, and financial arrangements. Once customized, the document can be filled out electronically, allowing for easy collaboration among stakeholders. After completion, it can be sent for eSignature, streamlining the approval process.
Steps to complete the clinical agreement template
Completing the clinical agreement template electronically can be done efficiently through the following steps:
- Access the template: Download the clinical agreement template from a trusted source or create it within your electronic document management system.
- Fill in the details: Input essential information such as the names of the parties involved, the purpose of the trial, and any specific terms or conditions.
- Review for accuracy: Double-check all entries for completeness and correctness to avoid any legal issues later.
- Request signatures: Use airSlate SignNow to send the document for eSignature, allowing involved parties to sign securely from any device.
- Store securely: Once signed, save the completed document in a secure location for future reference and compliance purposes.
Key elements of the clinical agreement template
Several key elements are essential in a clinical agreement template to ensure clarity and legal compliance:
- Parties involved: Clearly identify all parties entering the agreement.
- Study objectives: Outline the purpose and goals of the clinical trial.
- Responsibilities: Define the roles and responsibilities of each party, including data management and participant safety.
- Confidentiality: Include clauses that protect sensitive information shared during the trial.
- Compliance: Ensure adherence to relevant regulations and ethical guidelines governing clinical research.
Security & Compliance Guidelines
When handling the clinical agreement template electronically, it is crucial to adhere to security and compliance guidelines to protect sensitive information. Utilize secure platforms like airSlate SignNow that offer encryption and secure storage for documents. Ensure that all parties involved are aware of their obligations regarding confidentiality and data protection. Regularly review compliance with federal regulations, such as the Health Insurance Portability and Accountability Act (HIPAA), to maintain the integrity of the clinical trial process.
Digital vs. Paper-Based Signing
Choosing between digital and paper-based signing methods can significantly impact the efficiency of managing clinical agreements. Digital signing, particularly through platforms like airSlate SignNow, allows for faster processing times, reduced paperwork, and easier access to signed documents. Electronic signatures are legally recognized in the United States, making them a valid alternative to traditional signatures. In contrast, paper-based signing can lead to delays, increased risk of document loss, and challenges in document retrieval. Embracing digital methods enhances workflow efficiency and supports better collaboration among trial stakeholders.
-
Best ROI. Our customers achieve an average 7x ROI within the first six months.
-
Scales with your use cases. From SMBs to mid-market, airSlate SignNow delivers results for businesses of all sizes.
-
Intuitive UI and API. Sign and send documents from your apps in minutes.
FAQs
-
What is a clinical agreement template?
A clinical agreement template is a pre-designed document that outlines the terms and conditions of clinical collaborations. It helps streamline the process of establishing agreements between parties involved in clinical research or trials, ensuring compliance and clarity.
-
How can airSlate SignNow help with clinical agreement templates?
airSlate SignNow provides an intuitive platform for creating, sending, and eSigning clinical agreement templates. With its user-friendly interface, you can customize templates to fit your specific needs, making the process efficient and straightforward.
-
What are the benefits of using a clinical agreement template?
Using a clinical agreement template saves time and reduces errors by providing a standardized format for agreements. It ensures that all necessary legal and compliance aspects are covered, which is crucial in clinical settings.
-
Is there a cost associated with using airSlate SignNow for clinical agreement templates?
Yes, airSlate SignNow offers various pricing plans that cater to different business needs. These plans include features for managing clinical agreement templates, ensuring you get a cost-effective solution for your document management.
-
Can I integrate airSlate SignNow with other tools for managing clinical agreement templates?
Absolutely! airSlate SignNow integrates seamlessly with various applications, allowing you to manage your clinical agreement templates alongside other tools you use. This integration enhances workflow efficiency and document management.
-
Are there any templates available for clinical agreements?
Yes, airSlate SignNow provides a library of customizable clinical agreement templates. These templates can be tailored to meet your specific requirements, ensuring that you have the right documentation for your clinical projects.
-
How secure is the information in clinical agreement templates created with airSlate SignNow?
Security is a top priority for airSlate SignNow. All clinical agreement templates and associated data are protected with advanced encryption and compliance measures, ensuring that your sensitive information remains confidential and secure.
Clinical trial agreement
Trusted eSignature solution - clinical trial agreement
Join over 28 million airSlate SignNow users
Get more for clinical trial agreement
- Sign Church Donation Giving Form online
- Sign Church Directory Form electronically
- Sign Children`s Ministry Volonteer Application online
- Sign Volonteer Application electronically
- Sign Credit Card Donation Form online
- Sign Fundraising Registration Form electronically
- Sign Award Nomination Form online
- Sign Online Donation Form electronically
The ins and outs of eSignature