Countersignature Commercialization Agreement Made Easy
Get the powerful eSignature features you need from the company you trust
Select the pro service made for professionals
Configure eSignature API quickly
Work better together
Countersignature commercialization agreement, in minutes
Cut the closing time
Maintain important information safe
See airSlate SignNow eSignatures in action
airSlate SignNow solutions for better efficiency
Our user reviews speak for themselves






Why choose airSlate SignNow
-
Free 7-day trial. Choose the plan you need and try it risk-free.
-
Honest pricing for full-featured plans. airSlate SignNow offers subscription plans with no overages or hidden fees at renewal.
-
Enterprise-grade security. airSlate SignNow helps you comply with global security standards.

Your step-by-step guide — countersignature commercialization agreement
Leveraging airSlate SignNow’s electronic signature any organization can speed up signature workflows and eSign in real-time, giving a better experience to customers and staff members. Use countersignature Commercialization Agreement in a few easy steps. Our mobile apps make work on the move possible, even while off the internet! eSign documents from anywhere in the world and close trades quicker.
Take a step-by-step guideline for using countersignature Commercialization Agreement:
- Log on to your airSlate SignNow account.
- Locate your record within your folders or import a new one.
- Open the document and make edits using the Tools list.
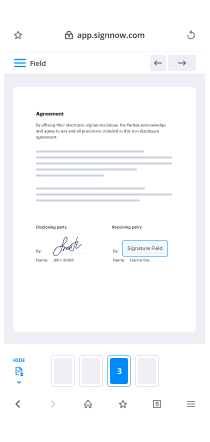
- Place fillable areas, add text and sign it.
- Add numerous signers by emails and set up the signing sequence.
- Indicate which users can get an executed copy.
- Use Advanced Options to reduce access to the template and set an expiration date.
- Click Save and Close when finished.
Additionally, there are more extended capabilities available for countersignature Commercialization Agreement. Include users to your collaborative workspace, browse teams, and track cooperation. Millions of users all over the US and Europe agree that a system that brings everything together in a single holistic digital location, is exactly what organizations need to keep workflows working effortlessly. The airSlate SignNow REST API enables you to integrate eSignatures into your application, website, CRM or cloud storage. Try out airSlate SignNow and get faster, easier and overall more productive eSignature workflows!
How it works
airSlate SignNow features that users love
See exceptional results countersignature Commercialization Agreement made easy
Get legally-binding signatures now!
FAQs
-
How do you countersign?
Suggested clip How to Countersign the Application Form and Photo - YouTubeYouTubeStart of suggested clipEnd of suggested clip How to Countersign the Application Form and Photo - YouTube -
What is a countersigned lease?
A countersignature is an additional signature that is placed on a document after it has already been signed. It is a way to provide authentication and confirmation. ... Most all contracts will have two signatures on them. The first party will read the agreement and sign if they are willing to take on the terms. -
What is a countersign contract?
Countersign (legal) From Wikipedia, the free encyclopedia. Countersigning means writing a second signature onto a document. For example, a contract or other official document signed by the representative of a company may be countersigned by his supervisor to verify the authority of the representative. -
Can you deposit someone else's check in your account?
You can deposit a check made out to someone else in your own bank account if the payee endorses the check over to you. They will need to write \u201cPay to\u201d on the back of the check and sign it. ... Some banks will accept such a check only if the payee is present when it is deposited, so they can verify their ID. -
How do you countersign a picture?
Your countersignatory should write the following on the back of one photo: 'I airSlate SignNow that this is a true likeness of [title and full name of adult or child who is getting the passport]. ' They must add their signature and the date under the statement. -
What to do if you can't get anyone to countersign a passport?
If you can't find anyone to do it, send a letter with your application explaining why you are unable to get a countersignature, and forward additional photographic ID such as driving licence. -
What is TC countersign?
Counter sign on TC / School leaving certificate shall however be submitted to the school positively at the time of admission. And In case, someone is migrating from different state within India, the Transfer Certificate (TC) duly countersigned by the CBSE Regional Office. -
What is the commercialization stage?
The commercialization stage of any New Product Development process is where the 'rubber meets the road' and the product you are manufacturing in China gets introduced into the market. This stage is actually the final stage of the development process. -
How do you sign over a check?
Suggested clip How To Endorse A Check To Someone Else - YouTubeYouTubeStart of suggested clipEnd of suggested clip How To Endorse A Check To Someone Else - YouTube -
What do you mean by Commercialisation?
From Wikipedia, the free encyclopedia. Commercialization or commercialisation is the process of introducing a new product or production method into commerce\u2014making it available on the market. -
Can you cash a check that's not in your name?
To cash a check that's not made out to you have it signed over in your name, by the person it's made out to. It must say "Pay to the order of NEW PERSON" then signed underneath. -
Do you need a countersignature for passport renewal?
Some passport application forms and photos need to be \u201ccountersigned\u201d by somebody else to prove the identity of the person applying. ... renewal of a passport for a child aged 11 or under. renewal of a passport if your appearance has changed and you can't be recognised from your existing passport. -
Can a Neighbour countersign my passport?
Who can countersign a passport form? ... They must have known the person applying (or the adult who signed the form if the passport is for a child under 16) for at least 2 years. They must be able to identify the person applying such as being a friend, neighbour or colleague (not just someone who knows them professionally)
What active users are saying — countersignature commercialization agreement
Related searches to countersignature Commercialization Agreement made easy
Countersignature commercialization agreement
good morning good afternoon wherever you are and welcome to the webinar balancing innovation and risk in the digital marketplace here is our little agenda I have two pleasure moderating this session and welcome that again and thanks for participating with me are Don Rosen our us sleep partner and Patrick Barnett who is the CEO of qumran our technology partner in this session my name is Rudy Blood Moon i'm the managing partner of lacp good to see a number of names that I have the pressure knowing personally as the topic we will address in this event is an evolutionary process there will be follow-up webinars and we will keep you posted please be informed that we record this session in the aim to give you the opportunity to share your experience within your environment please use the question box at any time during the presentation and demo for the Q&A part towards the end of this session so let's get started you certainly remember what type of speed steel was introduced electricity automation it took yours today that we will look at what speed we are confronted with and what it will mean you my kids to call for a completely different strategy in operations in communications distribution in other words very different business models all technology enabled and this is why we have asked a provider to participate in this event showing an example about how to reach out to individual profiles and the masses other questions will come up for example a what is then the difference between an e record and evidence in the digital world the other legal questions what privacy for the new comfort zones etc so and i would like to hand over to Dan Rosen and as I said he is our lead partner and to set the scene dawn over to you thank you really that's really said the world is changing at lightning speed and it is a disruptive change take for example the mobile revolution would start with the launch of the first iphone in 2007 the previous market leaders blackberry and Nokia are either non-existent or barely existent at this point today more people access the internet through their smartphones than through pcs or any other mechanism the rise of facebook has been dramatic it is now the most visited site in the US and actually surpassed google recently even though it creates no content Airbnb is the world's largest accommodation provider it owns no real estate but it offers more beds than the hundred-year-old Hilton group uber is the world's largest taxi company but it owns no vehicles Alibaba is the largest market cap retailer and that's no inventory you can say that these disruptive changes happened almost overnight certainly compared to steam or electricity and every other night a new company is changing or attempting to change in industry next slide please is this true of the health care industry as well we believe it is I think it's obvious that there are major transformations occurring as health care reforms encourage consumers to play a bigger role the industry has been traditionally business-to-business but it has to move to a business to consumer model even though most companies are dragging their feet in doing this they've been slow to join the digital revolution and that is a problem when customers expect health care to be like any other industry where they can have a highly personalized experience and they can use the tools that they're familiar with another industry is to evaluate their options there are new entrants in the industry or aware of this people like Walgreens retailers technology companies like Apple they're arriving on the same prepared to disrupt health care and get a larger share of the health care spend next please another example of disruption in new industries or traditionally old industries is the timekeeping industry switch watches were sold in 2014 a total of twenty nine point two million but Apple when they introduced their Apple watch sold over a million watches in the first 24 hours Apple has incredible customer loyalty and a focus on satisfying customers and relentless improvement of their products then this is paying dividends enormously Apple is not one of the most profitable companies in the world next please more than 70 percent of patients who are online in the United States using internet to find healthcare information and more than forty percent of the people who diagnose their conditions through online research how to confirm by a physician people are using the internet they're using the media that they have available them to decide how to manage their health the more that healthcare data becomes digitally accessible the more patients will use it to weigh and potentially reject expensive health care treatments this is particularly true in the United States where patients pay a greater percentage of the cost of their drug therapies 25% is not unusual then they do for other health care expenses such as inpatient services not surprisingly consumers are demanding more information so they can apply the same cost-benefit analysis and research techniques they use to purchase cars or phones when they purchase health care they're also making more informed rational choices about where they put their money if companies don't join this digital dialogue and influence the conversation to lose an opportunity to shape it and then maybe put on the defensive trying to refute the statements based by those that do take part next please health authorities are moving to create data liquidity and that means that they and others who have data available large pools of data whether it be big data repositories or simply outcomes data will control a large portion of the data that's relevant to the pharmaceutical industry which means the pharmaceutical industry will control less of a building trust and our authentic relationship between drug makers and patients will require sharing the bed with a good for example when real-world evidence points to adverse events or diminished advocacy or response rates in a particular patient population this information needs to be communicated public in without delay next slide please patients are paying more for drugs they switch to high deductible health plans and they face larger out-of-pocket costs for specialty products and the divisions between pharmaceutical R&D the Food and Drug Administration approval and commercialization are blurring pharmaceutical companies can't afford to be proactive and not to be proactive as patients in health plans negotiate access to their products putting drug costs in the context requires access to patient data and evidence connecting drug intervention with patient health outcomes evidence generation has to continue after a drug receives FDA approvals clinical safety and efficacy measures get way to real-world performance and demonstrated patient outcomes consumer expectations in health you have shifted in the last five to ten years due in part to experiences in other industries easy access to health information increasing costs and a desire to understand a physical and biological level what's happening in their bodies has led to a stronger opinion about how where and when to deal with it illnesses next please there is regulatory movement as regulators are exploring new ways to integrate patient experiences into drug review decisions and patients are pushing for further inclusion into these processes now this is problematic from a regulatory standpoint for example most comparative effectiveness studies and costs that that Miss data can only be shared of an insurer or health system as the pharmaceutical company to provide them according to the current regulations as competition for market share increases the ability to produce and communicate research showing that one product works better than another or saves money while providing a desired health outcome if the powerful message to insurers and providers and as a benefit to patients but FDA leaders are concerned that financial incentives cloud industry research and promotional messaging so even though there are steps under way to change the regulatory landscape so that it is possible to provide information about value there will be an ongoing concern about the validity of the information being provided and consumers may be pursuing an agenda that can run counter to conventional methods of drug development and approve approval I mean demonstrating for example that a tumor shrunk or that a biomarker was successfully targeted by a drug during clinical trial doesn't necessarily in a patient will feel any better in chronic obstructive pulmonary disease for example the ability to accomplish daily tasks are just lifting children are using a hairbrush without succumbing to breathlessness can be more important to patients than a clinical pathetic to see number and this kind of information has to be available to patients and making their decisions next please life science companies are still on the sidelines in terms of digitally enabled healthcare they need to be at the forefront of developing beyond the pill service of deliberate values to patients but despite access to unprecedented data and technologies that can be used to drive better health outcomes by influencing customer behavior few companies are really exploring digital engagement models the opportunity to learn more about consumerism developed better more targeted products and services far outweighs the threat digitization presents the companies for now but unless incumbent pharmaceutical companies move quickly innovative competitors such as walgreen or Apple may grab a greater share of benefits and the strawberry sense of customer loyalty next please so what are the implications for life sciences patient organizations formed around specific disease areas have a key and understanding of the issues that matter most that can offer valuable insights to inform clinical trial designs and protocols biopharma company should also consider the various roles new entrance according to support patient organizations and to drive consumer engagement and be involved in these in may clinical trials gov listed 343 open clinical studies that included at least one patient recorded outcome measure according to a nature re analyst it's nineteen states have passed right to try laws in reaction to patron demands for access to new experimental therapies before the FDA declared safe for general use so capturing evidence in an increasingly granular level with respect to nuances in patient populations along the lines of gender ethnic origin environment consumer habits will deliver new insights and how to specific patient populations respond to therapy the purpose of this webinar is not to tell you how to deliver information but rather that given the need to increasingly communicate with the public in non customary ways you're going to need tools that better capture these interactions so today's reality is that there's a balancing act for organizations between being successful and putting your business at risk there are a hundred thousand rules and regulations worldwide and these are growing every day companies a face with the year-to-year increases in their compliance budgets at the same time the world is going digital and you need to adapt rapidly to the digital health revolution most US CEO see high values and digital technologies that give better and safer customer experiences the top three priorities of us ceos are customer experience data and data analytics and digital trust next with digital technology trends are impacting all the relevant stakeholders and the interactions between the industry regulators and patients and providers certainly aging regulatory processes are hindering industry's ability to work productively with regulatory agencies in the new health economy new regulations with relevancy for engaging digitally are impacting all relevant stakeholders including the regulator's industry and patients and providers then consumers want to provide it to put into developing therapies and they know that the information the author is being utilized industry wants to innovate in using this but the current state of the regulations is going to impede that to some degree and certainly require that companies are extremely careful and balancing their need to innovate with their need to satisfy regulatory compliance strictures next please the are just an overview of the existing regulations which do impact digital engagement from the FDA regulatory requirements for post marketing submissions of interactive promotional media to the expected to become final internet social media platforms regulations legal considerations from other organizations and industries new legal rules in the EU regarding privacy regulations and rules in the EU about information to patients scope content channels controls from additional debt cetera these are all regulations that are under review they're in a state of flux flux but the industry has to be prepared to manage their adherence to these rules while at the same time innovating next please as an example you have the UK MHRA initiative which requires that market might you know authorization holders regularly screen internet or digital media under their management for potential suspected adverse events and report on these as spontaneous reports next please along that lines here's an example of using social media as a data input source now 35 to 40 percent of postings for treatment in health-related for include potential ADR mentions only about a half a percent of these include a drug name and also a potential ADR however with 615 million users 58 million tweets per day this can result in a considerable number of potential adrs the challenge in analyzing this data is that there's little context little structure and the language venues and their misspellings and query limits and different terminology used for the same drug in multiple cases so one of the potential solutions for this was the use of sentiment analysis on the repositories of information in Twitter for example some of our team did a first attempt to demonstrate the feasibility of using sentiment analysis using digital media monitoring to gain insight into the clinic we use profile to recently proved melanoma treatments the high level analysis showed the sentiment analysis has the potential for showing differences between the two selected test products as well as information about similar treatments the challenge in doing this of course is to gather data to mine while achieving and demonstrating regulatory compliance and that is a segue into our next session Rudy back to you well thank you don for showing part of the scope that we all as a society in 23 and regulators are faced with many opportunities not only regulatory with business that encouraging is encouraging in this environment where there is sun there are challenges as well review this session together with your team and we will come back in the question and answer session so let's look at em a potential technology provider come from AG over to you Patrick Thank You Don and thank you rudy for having the opportunity to be here with you on the webinar and to present them kumrah good morning and afternoon wherever you are my name is Patrick boarded I'm the CEO of conglom which is a provider of web archiving social media records management and session replay solution in the following section I will present to you how come Ram is used by healthcare organizations they more specific custom use case and wrap my part with examples from other industries Qumran provides a solution for session recording and reply across digital channels like for example web mobile or even social media the arrow of use is grouped in three applications first to ensure compliance second and uncreate evidence second is to detect and prevent fraudulent behavior across these channels and lastly to improve the customer experience and gain deep customer insight in healthcare we have the following experience with the University Hospital of Zurich we record user access to Oracle house platform and we create proper and visual evidence for accessing critical patient data this is important for the university to ensure that their modern patient platform is secured and the patient data is safe another customer of us which is a clinical hellz who provides with clint paul a digital clinical trial platform we ensure regulatory compliance but also improve the customer experience and provide deep customer inside this is the case we will show in a demo and just a little bit later let me give you a bit better understanding of klimpaloon what cliff Paul is doing klim pal provides a platform for electronic informed consent for clinical tries their intention is to transform clinical trials to be more modern efficient and patient centric today the platform is already transforming clinical trials and is in use in multiple studies ranging from very small with 50 patients to very large with 10,000 and more patients clin Paul has been adopted by several organizations again ranging from patient recruitment companies and research institutions to several top 10 pharmaceutical companies to examples of clinical trials are very cold trial for mend or medical devices and a trial for sanofi deliberately asked my quest which is running at the moment to give you the context about the clip pal demo let me first give you the goals of the partnership between a clinical house and come run and frame the demo you will see shortly the challenge II clinical hell's faces is exactly what you heard from dog before it's balancing the regulatory requirements and additionally gaining patient insights we call that complaint insights additionally to the audit trail Clint Paul wanted to have evidence along the process and across the digital channels the process starts as you can see in the bottom starts with study campaigns like the one from verrico on facebook which attracts the patients on the clinical trial info page where they can pass a pre screener at the end clin pal wants to gain more and deeper insight about the conversion rate of patients joining the info page and going through the pre-screening these two parts will be shown now for that I going to switch my screen I'm going to show a demo which was recorded before the session so that we can look to a video instead of doing a live demo here so what you can see here this is the dashboard of claim Paul here in that example this is for the doctor so what he sees is that the patient has requested to join the trial this is our CTO Simon shorter so this is just a demo page with demo data and not real data because as you understand we can't show that for data privacy reason so what the doctor here can do is he can show khammam evidence and see what the user been studying on the website on that info page and then also what he filled out on the pre-screen so what you see here is on the top this is the common room solution it looks like a video player and what we're doing is weary replaying the session from Simon sure the user which was part or which took part of the pre-screen so you see how he's moving his mouth you see what kind of information he's looking at and what he's reading and also the information he's not looking at this is especially important in cases where there is important information which need to be read and also agreed on so that you can prove that the person was informed about potential risks this is the info page right of claim Paul it's the demo system you see now here the user clicking to take part in to that clinical trial study so the next phase is then he's on that pre screener it's a large forum which goes through multiple qualified questions to qualify the patient for the the study the trial here so again you see how he is moving through the application you gain a lot of insight also about your application and the entire customer experience here in that case you see where he is hesitating like the irregular medial schedule he was resid has it between years and no and you gain again a lot of a deeper customer insight about his specific behavior our solution goes even so far that we're recording every keystroke and we can see where someone is changing text or is doing any kind of mistakes the solution is not only used to improve the customer experience and to create evidence for compliance reason but often is also used in fraud cases where it's to document and detect an even prevent fraudulent behavior of users so again sessions going to end here rad you see the person typing in all his information and we're going to see in the other demo that if he would know stop and and and stop the session and not send the information then he will be a lost patient so what the solution additionally provides is analytics capability like heat maps where you see in a visual individual where the person was clicking or where the person was moving with his mouth so we can aggregate that data through all your patients which went through the pre screener which gives you a good overview on where people are hesitating where people are clicking where people are looking at in your application let me give you another example another demo this is again clin Paul this is about Clint Paul to gain deeper inside into the customer behavior and also getting more analytics around where they lose the patient in their in their entire conversion from entering the info page and then going through the pre-screen so what we've been doing here is a funnel we define the funnel say a person joins the splash page info page we see that this is just pure demo data 24 joint and we are seeing that we're losing four of them which is sixteen percent and then the form was dissected in multiple forum section and we're seeing in that funnel where we're losing the people so this is highly valuable for claim Paul because it gives you it gives them an immediate overview on where they stand on the conversion from bringing patient attracting patients on Facebook through campaigns on their info page and then also qualifying them for the tribe so in that example right we're going into the irregular meal where we've seen before the person which was hesitating about regular meal and we see that we're losing two sessions there so we can click on the forum section we see now two sessions or 12 sessions continuing and two sessions which we're losing and now what we can do is we can do the same thing we can open the session and relook at the session review the session and see what the patient was doing and where he was I'm stopping again demo data you're gonna see rights hero boarded being my son here so and this is just demo data what you see is that he fell dog almost all the information and at the end he decided to not to take part of the clinical trial not being part of that study here which somehow is a shame white because he was a perfect patient he filled out all the right questions and and it somehow lost so what you can use common room to is you can gain insights go back and re target these people right for a late to study or even for the same study and to go back let me go back to the slides and share the slides here as you saw we're recording all the information but also user interactions to document who so what this is relevant to ensure regulatory compliance across all your digital channels we provide also the capabilities not only in web or mobile but we provide also the capabilities of social media records management what we do is recording also all social engagements and that can be twitter linkedin instagram or or any almost any other social media platform you can envision and what we can do is create evidence which can be archived and preserved for a long time long time 7 10 years or longer I think important is to understand the difference right between social media monitoring and social media records management like Don was explaining in the case which was done by LCP to use social media monitoring sentiment analysis to analyze kind of at the data to read something out of it don't also mention that difficulty is right to create the evidence and to record all these data somehow why because someone can easily delete his content from Twitter and then it's what you're missing is having kind of the proof point that there was a data out there like i was saying we're not doing sentiment monitoring or sentiment analysis but just the word just a recording tool to create the evidence the main case story here is to govern what all the employees of a company do and on all the social channels posting commenting and and and all the other things right including extraction and feeding into and the analysis is what we can provide this is key to react before an alert even happens so look out for this topic because we gonna have a specific webinar to come together again with our partner LS CP here and and we're going to come back to you also for digging more into that details and we're willing to do also in all sessions comm room has many customers especially from other industries mostly regulated industries like banking insurance and government let me give you three examples right on how we help to ensure compliance prevent fraud and improve the customer experience the Swiss Federal foreign agency the FDF a is the first agency to achieve compliance with the swiss federal audit oversight Authority there's a policy for web and social media and how you need to keep that for the future but additionally benefits for fgfa is to retain invaluable knowledge in their global and constantly changing digital environment for them it is very important that they can record and store that information to go back in in the future and see what they've been publishing in the past you base is a different case UBS case is all about fraud detection and Prevention for customer identifying data they have to protect their most critical data which is all related to their banking customers so Qumran records globally all-session accessing any kind of customer identifying data from internal employees and does an analysis of potential fraudulent behavior with a business intelligence solution to prevent exactly fraudulent behavior but also to protect people right for being accused wrongly about potential fraudulent and cases the last one around improving customer experiences is about a health care insurance called CSS here in Switzerland it is about better customer service and they've been able to achieve that due to fifty percent reduction of the average customer service call duration and what they also achieved was a sixty percent increase of first call resolution that led to tremendous lower costs right due to like the customer was some stating 6,000 hours efficiency gain every month so handing over back to you Rudy for the rest of the webinar today well thank you patrick and i think that was an interesting view on a specific topic that can be considered part of the patient recruitment informed consent as being a module of clean pal but am I correct Patrick that quorum is an integrated part of twin town yes we're a part of their solution so where they are using us to record their platform and and absolutely were part of their entire offering and the customer the user doesn't see any difference and coming back to your slide about records management we understand that for each archiving element you have an audit trail like it's outlined in partly level yeah so what we are normally doing in these cases is we're recording what's happening on the social media channels and because we want to leverage the investments the customer already been been doing in the past right all of them normally use a potentially even a DoD certified records management solution or a CFR part time 21 kind of tulum solution so what we're doing is we're integrating the content properly in these records management system so that they have one legal archive and not creating another silo and that adds tremendous value to all customers because not only its foster being up to speed but it is right they have one single source of truth which is their core back-end system and this is what we've been doing for in-house care insurance here integrating as an example into IBM filenet or in 20 / txt records management but it can be any kind of solution like emc documentum or other ones used by the customer we will get to that method are in a special that part of the webinar in a special session but let's take a moment to look at some of the questions that have come in at one very attentive participant is asking I would come on avoid Nestler event in Europe you act as a virtual and global compliance tool and how would you go about let me probably explain what they hunt in the potential Nestle went is I assume it's it's what's actual in the new was right Nestle had a campaign in Germany running on Twitter where they had a hashtag ask Nestle and they opened kind of themselves to the entire public and said you can ask whatever questions you want to ask and we're going to give you answer unfortunately that turned into somehow right you can call it a shitstorm why because the people started to ask why are you killing rainforest right why are you supporting child work right why are you on the palm oil right them why are you doing that and so what's happening in that entire Twitter feed again go look for hashtag ask Nestle you got to see that there are a lot of aggressive tweets out there and and at the end Nestle is answering almost in real time in a very good manner I need to say they do that really really well and and but this is a direct open communication right um and it is relevant it is relevant what Nestle states there because that could be used again against them so what's important there is one is you can do sentiment analysis but it's obvious if you see the content right what the sentiment is but what's more important is to record that so that data doesn't get lost so if a user would delete is his tweet as an example right you count anymore recognize where everything started and and that's the important one that's exactly what I've been talking and we're gonna dig into it in the webinar is really about social media records Thank You Patrick the next question comes right into sentiment and pharmacovigilance could you please providers and the link to the sentiment approach people to evaluate participation in a pilot yes and please provide us with your contact details and we will facilitate the contact with dr. Viet Midler the next question what would the LCP contribution that be in a way forward Don would you like to answer this one yes certainly that would depend on where your social media initiatives stand already in particular where your effort stand in regard to regulatory compliance with your evolving social media initiatives our domain is process and content in a regulated environment so we would work with you in evaluating how you're using social media you know and how you can most effectively use the tools and processes we recommend to ensure regulatory compliance while still being able to leverage digital media effectively and we do that based on where you stand and helping you to put together a vision and strategy for moving forward with this if you are interested we can certainly discuss this further and discuss how you can use technology such as chrome RAM in meeting regulatory requirements Thank You Don and would that be a possibility to talk to one of your current customers for example ups with its global approach Patrick that's for you it looks like you base being one of the logos we've been shown yeah absolutely so when you base is not only a reference customer right it is a very important part for us um and also to evolve so we we have multiple reference customers we can also provide references out of the health care market or even provide references from the industry analysts like Gartner so absolutely if you wanna I approached me and if you want to have more details we're gonna share our contact details at the end of the webinar so feel free send me an email getting contact with with myself Patrick or or with Rudy or with Don and we're happy at provide further information also on the references the next question I would be interested in business intelligence can we talk at one of these days and with whom after day but I absolutely so again right I think the best way to address that question is reach out to to Don reach out to you Rudy or two myself you're going to see the contact details at the end of the webinar here phone and an email and we're absolutely happy to come back to you and set up a call with with with come home experts and also L SCP experts and and no thanks for that question I'm we're excited to have the opportunity to talk with you about that go and we have corporate information governance in progress who at what kind of criteria that would we look for to fit this into Donnell is that a question you could answer that would be one that would require a fairly long and extensive discussion so if you'd like to pursue it further my contact information is practice there will be at the end of this and I be happy to have a discussion with you about thank you there is no regulatory pressure at the moment what why should we become active then I may take that go for it i think it's a very good question right um we see there is a higher definitely higher regulatory pressure in other industries and like Dom was presenting on one of slides right you see a lot of regulations coming out or just been published recently so i'm heavily interested in your view early what what are you answering well in our discussions with customers we recognize that this part of the game people tend to look at more from a regulatory side our recommendation is to look more at the business opportunities getting closer to the patient listen to the patient's voice because over time it will become more and more important to recognize an archive patient outcome this will help to determine the right price you are asking in the marketplace and therefore come back to us discuss what kind of opportunity we could work on together I fully agree with you on that I still would go back also about the risks right you see it with Nestle this is not only about the regulatory side this has also a lot to do about kind of managing and hatching the risks and within the company so about eternal policies society opinion for example death so here we have a little menu about where Qumran technology functions that can provide benefits not just for regulatory as I just said but for innovation purposes and one example that is here in conjunction with clin path so what could be a next step from a more specific offering we have a I after a workshop that we would elaborate the opportunities with you and understand together that what is important to you and focus on that for the next step then for example in a digital lab where different functions could experience what this would mean and how would you keep control so we have come to an end of this webinar and as we say here and please stay tuned for more specific webinars to come and you find the contact details either from Patrick or from me and Nick Cannon of course instantly provide also the contacts of dawn thank you for participating today and we look forward having you through with us take care bye bye thank you very much Rudy and on for having the opportunity to be on the webinar today with thank you bye thank you bye
Show moreFrequently asked questions
How can I eSign a contract?
What type of field allows me to eSign my PDF with my finger?
How can I add multiple signatures in several places in a PDF?
Get more for countersignature Commercialization Agreement made easy
- Print signature service Product Order
- Prove electronically signing Employment Contract Template
- Endorse digi-sign Project Management Proposal Template
- Authorize signature service affidavit
- Anneal signatory DJ Contract
- Justify eSignature Photography Quote Template
- Try initial Hotel Business Plan Template
- Add Incentive Agreement digisign
- Send Security Proposal Template electronic signature
- Fax Alabama Bill of Sale signed electronically
- Seal Performance Review Self-Assessment Template sign
- Password Sales Contract electronically signing
- Pass Computer Repair Contract Template mark
- Renew Sublease Agreement eSignature
- Test Power of Attorney Form autograph
- Require Car Lease Agreement Template digital sign
- Comment person digital signature
- Boost boarder countersignature
- Compel subject electronically sign
- Void Auto Repair Contract Template template signature service
- Adopt bill template countersign
- Vouch Medical Invoice template sign
- Establish Tourist Transport Ticket template initials
- Clear Volunteer Agreement Template template eSign
- Complete Theatre Press Release template eSignature
- Force Roofing Proposal Template template esigning
- Permit Pet Grooming Registration template digisign
- Customize Promissory Note Template template electronic signature