eSigning Substantiation Made Easy
Get the robust eSignature capabilities you need from the solution you trust
Select the pro service made for pros
Set up eSignature API with ease
Collaborate better together
Esigning substantiation, in minutes
Decrease the closing time
Keep sensitive information safe
See airSlate SignNow eSignatures in action
airSlate SignNow solutions for better efficiency
Our user reviews speak for themselves






Why choose airSlate SignNow
-
Free 7-day trial. Choose the plan you need and try it risk-free.
-
Honest pricing for full-featured plans. airSlate SignNow offers subscription plans with no overages or hidden fees at renewal.
-
Enterprise-grade security. airSlate SignNow helps you comply with global security standards.

Your step-by-step guide — esigning substantiation
Leveraging airSlate SignNow’s electronic signature any business can enhance signature workflows and sign online in real-time, providing a greater experience to clients and employees. Use esigning substantiation in a couple of easy steps. Our handheld mobile apps make operating on the run achievable, even while off the internet! eSign signNows from any place in the world and make trades in less time.
Follow the stepwise instruction for using esigning substantiation:
- Log on to your airSlate SignNow profile.
- Find your record within your folders or upload a new one.
- Access the record and make edits using the Tools menu.
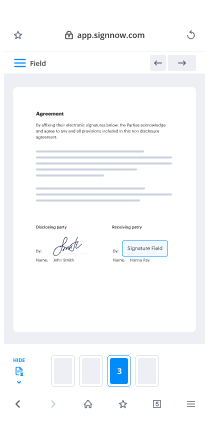
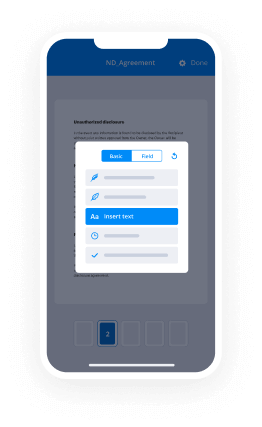
- Place fillable areas, type text and sign it.
- List numerous signers by emails and set the signing order.
- Specify which individuals can get an completed doc.
- Use Advanced Options to reduce access to the document and set up an expiry date.
- Press Save and Close when done.
Furthermore, there are more advanced features available for esigning substantiation. List users to your collaborative workspace, browse teams, and track cooperation. Millions of customers all over the US and Europe recognize that a system that brings people together in one cohesive workspace, is what companies need to keep workflows working smoothly. The airSlate SignNow REST API enables you to integrate eSignatures into your app, internet site, CRM or cloud storage. Try out airSlate SignNow and get faster, smoother and overall more effective eSignature workflows!
How it works
airSlate SignNow features that users love
See exceptional results esigning substantiation made easy
Get legally-binding signatures now!
What active users are saying — esigning substantiation
Esigning substantiation
hi this is Aaron Tish I'm the managing director of CSG I'd like to start off by saying thank you to all of you for joining likely you're all in your home offices we have a little over 150 folks participating in a call today we had over 200 sign up so I expect it will have more trickle in I hope you're all staying safe and healthy during these challenging times I'd like to introduce my colleagues that we'll be presenting today abigail what sick is a senior regulatory consultant at TSG abigail leads our federal regulatory team dr. Christine Green is a senior scientific consultant at TSG and a key expert in our animal Caribbeans team before abigail and chris start their presentation for those of you that are not familiar with TSG I wanted just to just say a few words about our firm we've been providing companies around the world with regulatory guidance and scientific expertise for over 30 years we help clients address technical and regulatory issues associated with taking their products to market in multiple jurisdictions we have offices in the US Canada and throughout Europe as well as partnerships around the world we are part of science group which is listed on the London Stock Exchange we serve a number of key markets in industry sectors including agricultural industrial consumer food and beverage animal health and medical teams are comprised of scientists and regulatory experts many of whom have previously held positions at regulatory agencies and in industry in fact Abigail came to us from EPA's animal CRO Viall division there's over 500 of us around the globe we speak over 30 languages we have 14 offices around the world and two dedicated R&D facilities we do encourage you to submit your questions through the presentation via the chat function we'll be monitoring your questions and we'll have about 15 minutes at the end of the presentation to answer your question also as we are navigating this webinar with presenters that are not physically in the same room so we think we've worked out all the kinks but please be patient with us so with that I will turn this over to Abigail to start us off thank you thank you and thank you all of us for joining us thank you for joining we want to start with a brief explanation of why we're all here for many years the market has relied primarily on chemicals to control pests devices have long been a felt small section of the market especially for insect control I think many are familiar with bug zappers however in the last few years we have seen an increased interest in devices for control of other pests such as much of micro organism devices providing non chemical means of controlling pests and consumers are always looking for alternative methods for pest control there is also an increased interest by consumer for supplementing traditional pesticides with the devices for use in controlling pests in hard-to-reach places however the regulations pertaining to the sale of devices can be confusing due to EPA's historically rather laissez-faire attitude towards devices a couple of the markets that is largely self regulated the goal of this webinar is to untangle some of this and to lay out the regulatory landscape of devices that mitigate pest we will focus many of our examples for today's discussion on antimicrobial devices but the gel but the general requirements we'll discuss today are applicable to all devices antimicrobial and conventional so this is the agenda that we'll be following today we will start with an overview of cipro we will discuss the definition of a pesticide device any pH device determination process we will discuss the jurisdictional boundaries between FDA and EPA and then the requirements for the sales pesticide devices in the u.s. principle and step in to discuss challenges and claims of sanshi a ssin with you before we end with our Q&A so cipro FIFRA is the federal insecticide fungicide and rodenticide act under this act the EPA has the authority over the sale and distribution of pesticides and pesticide devices in the u.s. everything we talked about today related EPA is regulated under FIFRA specific to today's discussion under FIFRA EPA regulates the composition testing labeling promotion distribution sale and use of pesticide devices in the u.s. I will note the devices are also regulated at the state level by a handful of states we will talk about that process in greater detail later in this presentation for now we'll focus on EPA as I mentioned for many years consumers have relied primarily on conventional chemical based products for disinfecting and sanitizing surfaces these products are registered as pesticides by EPA any of you who may be familiar with chemical pesticides will already know the registration process can be lengthy and expensive applicants must address a wide variety of data requirements and administrative forms they must provide a label for agency review they must pay a fee for service at the time and cost that can vary widely and at the end of all of that they end up with a notice of registration issued by EPA pesticide devices on the other hand are regulated but are not registered by EPA so what does that mean no administrative forms no data submission requirements no label review but there are labeling requirements and there are limitations than what you can and can't say and there are requirements for import and export it is these regulations that we'll focus on today to better understand what is required of you before you manufacture sell and distribute pesticides device in the US we will also talk about what isn't acquired but what are some additional steps you can take that's more confidence that your products will not run afoul of any EPA rule so before we can talk about what a pesticide device is let's figure out what a pest is an organism is declared to be a test under quote circumstances that make it dilatory us to man or the environment common examples of tests include insect leaves and rodents specific to today's discussion the definition of a pest also includes fungus bacterium virus's pram prions or other microorganisms living where you don't want them to be also for discussion today pest control under the authority of the EPA are those in the environment not microorganisms living in or on humans those organisms are regulated by FDA they're outside of the scope of today's discussion so we will talk about more about that jurisdictional boundary so what is the device a device and any instrument or contrivance intended for trapping and destroying repelling or mitigating any pests or any other form of plant or animal a device does not include equipment for application of pesticides such as tamper resistant bait boxes when sold separately from the Dybbuk from the pesticide devices are not required to be registered under FIFRA section 3 for those of you who are interested the regulation of devices worthless were defined excuse me in the Federal Register in November of 1976 in our extensions towards physical control of these of pet these include guns products that dependent primarily and user performance such as flyswatters mousetraps and as I mentioned application equipment so let's talk about some more specific example we'll start with the ultrasonic mouth propeller let ground vibrators used to repel Gophers or sound generators used to repel pests such as birds ultrasonic mouthed repellents emit the sound at a frequency that humans cannot hear but mice can't it drives me crazy and they run away pure non chemical pest control another example drinking water filter units the filter the filter reduces or eliminates microorganisms from the water using a physical barrier that captures when we are discussing filters I do want to point out an important distinction as long as the filter is using a purely physical means to remove the pest it is a device however if the unit contains any substance intended to disinfect the water then the unit is a pesticide and that must be registered another bit more complex example are salt chlorine generators a boat chlorine generator makes hypochlorous acid by using table salt and electrolysis the salt water passes through an electrical current the salt water is converted by hypochlorous acid until you have a quiet and those chemicals are used as sanitizers and things like women tools the generator is not sold with a pesticide but it is used for pest idle purposes so it's a device the key difference here is that the generators can use any normal table salt and be effective if a company sells salt for the expressed purposes of use in such a device then that salt will require registration as a pesticide so as you can see the details of how you sell these types of products can really make a difference to make it more complex EPA's opinion and guidance does evolve ion generators for example used to be regulated as devices but the agency's policy evolved and in 2007 they clarified the key distinction between pesticides devices the distinction was whether the pesticidal activity is due to physical or mechanical actions or due to a substance or mixture of substances the policy was related specifically to ion generators which the EPA declared is no longer devices but pesticides ion generators that use electrodes for example of copper or silver emitted ion mid ions when a current is passed through the incorporation of the electrode is what gave the ion generator its efficacy as such ETA determined that they acted as pesticides because the released ions and they did not destroy paths in a non chemical manner so it's all getting rather complex water filters we can all get purely physical means definitely a device but chlorine gas ion generators is that really physical it all starts to get very confusing and you don't want to get it wrong so EPA's given us something to help figure this out under the pesticide registration Improvement Act which is the fee-for-service program under which EPA obtains the resources to register testified the agency has a review category for determining whether fifth row registration is required for proposed product this category includes the determination of how EPA views your pesticide device and whether or not if it is a device or if it is a pesticide requiring registration this seat height category is optional you are not required to submit but it is a nice insurance policy when you aren't really sure whether or not EPA would agree that your product is the device this can give you an answer in writing one way or another for better or for work but be warned that you may not like the answer this category has an EPA decision review time of four months and 21 days and an EPA registration service fee of two thousand four hundred and eighty two dollars you can apply for up to a 75% reduction in this fee if you are a qualified small business the application will need to include some administrative forms a complete copy of the label for the device and a statement of all claims to be made on the product as well as its detailed written description of how the product works to kill destroy repel or mitigate a pest lastly you'll need to submit a schematic diagram or detailed engineering drawing diagrams flow diagrams or patent information we have mentioned it a bit but before I move on I did want to talk about application equipment according to EPA pesticide application equipment that is sold separately from the pesticide itself is not a device or a pesticide APA gives the example of a sprayer for a lawn herbicide that is sold separately from the herbicide which is considered to be application equipment which EPA does not regulate another example would be a fogging machine in which you pour a hydrogen peroxide solution like a spray bottle the machine is a way of applying the product as long as you sell the hydrogen peroxide separately then it is application equipment and not a device I next like to talk about the jurisdictional boundaries between FDA and EPA EPA has authority over products which claim to control pests in the environment FDA so the authority over products which claim to control pests in or on humans or over instruments which are either introduced directly into the human body either in contact with the bloodstream and normally sterile areas of the body or Khan excuse me contacts the intact mucous membrane but which does not ordinarily penetrate the blood barrier or otherwise enter normally sterile areas of the body medical devices require pre-market approval with FDA pesticide devices are really regulated by EPA sometimes this jurisdictional boundary gets very close and for this reason you'll see some very specific language on products for example a UV device used to disinfect surfaces in a hospital room it's going to be regulated by EPA however that same device used to sterilize medical equipment for surgery would be regulated by the FDA okay so we've determined whether or not what you have is the device if it is what next we're next going to talk about the requirements for sale of a pesticide device in the US we will be talking about labeling important exports as well as state registration well just give the slide requirements for pesticides advice is defined by EPA to start you cannot make any false or misleading statements you cannot say that the product does something but it does not actually do this includes efficacy how do you prove it critically to talk more about with more about that with you next but short to say you shouldn't have data on file demonstrating at the product is application et does not have a performance standard for this data but you do need to have data your label must include the EPA establishment number this means that the facility that produces the device must be registered with EPA the process to register a facility is a pretty straightforward table or process there are annual reporting requirements for pesticide and device producing establishments but no fee please note that establishment registration is required whether the facility is foreign or domestic each establishment receives an alphanumeric code for the facility and the number for the last facility to produce the device needs to be printed on the device label the producer must register the establishment reports and production annually heat books and records and subject itself to EPA inspection tessai devices are also subject to child resistant packaging requirements if the product meets certain toxicity special important export importers must file a notice of arrival with an EPA regional office this may be filed electronically through custom and Border Patrol's automated commercial environment or 8th system please note that this must be filed before the device arrived a copy must accompany the shipment do not wait to file this until your device dries or you will be stuck with a device that is not able to be fully imported exporters must use prescribed export labeling the most important thing is that the labeling must be in English and a language of the country of import and otherwise follow the labeling requirement I've already discussed we mentioned state registration in addition to different regulation device registration is required in eight states listed on the slide mr. Colorado Hawaii Indiana New Mexico Oklahoma West Virginia and Wyoming and for the purposes of this discussion we promoted DC to the list of state different states have different registered registration requirements and they change in time to time Indiana used to require submission of the actual device itself and away from that as a requirement so they still do reserve the right to ask for one but most states aren't looking for more than your application form and your money so you'll want to engage with your regulatory staff or your consultant prior to making any submissions for six states device registration takes about 30 days for the other two typically about six months so Indiana can take even longer up to nine months the data review is required the cost to register one device in all eight states is approximately $2,500 so we know what a device is we know what we need to do to sell it in the US but as you can tell there are challenges we talked about applicators and whether your product requires any different oversight at all or whether you're applying a fifth press we spoke about how to determine if your product is a device or not by using pre Fe category and zero zero zero nine we talked about EPA and SBA and whether your product is its device a pesticide device or a medical device and we touched upon the label claims and misbranding you need to have data unfiled demonstrating that the product does what you say it does if you want to say that your device is a disinfectant there isn't any performance standard for that term for devices your best strategies to mimic the performance standard for standard disinfectants to the best of your ability to that end I'm now going to turn the floor over to Chris for her to talk to you about claims and fancy aja how do you sold your products as efficacious as you say it Thank You Abby so what we typically find is that most companies are unaware that the claims that are making on their website or in their marketing materials actually considered deceptive or false advertising in the eyes of the EPA in most cases companies will see an immediate need that they feel that product can speak to the printing in order to appeal to these customers who are really just seeking a solution in a field where there often isn't an easy answer we're seeing it happen right now with the kovat 19 pandemic but what the companies don't realize is that the tests that are that they have on file or that they have done already Ruby does not support the statement that they are now making or trying to make in their in their new advertising so for example an environmentally product that claims it's biodegradable when in fact only components within the product are biodegradable or making it a health claim such as protects against quoted 19th a product that kills viruses from a surface you can't make a public health claim like that and what you would need is a lot of toxicological studies as well as some clinical trial data to support a public health claim such as a curing a disease of some sort and then there are blanket statements such as kills in 30 seconds or safe and effective these are blanket statements that are made without any qualifying remarks so in other words you can't say it kills in 30 seconds without qualifying the statement regarding to the the organism that it applies to and then exclude all the longer kill times that are required for all the other micro organisms so that's that's considered deceptive now then there's a reason why the this is a problem first next slide please first it can be difficult for consumers being false advertising claims and from true ones and many companies will leverage this special claim in order to support selling their product at a premium so in other words people are paying for expectations that a product that will not necessarily meet but more important to gather those is that the claims often can have an impact on public health so in an effort to help ensure that consumers are provided with accurate information and to protect human health government bodies are united in taking measures to protect consumers from deceptive practices and false advertising pesticidal devices these government agencies are both the EPA and the FTC or the Federal Trade Commission now the EPA has guidelines for pesticidal devices specifically in section 2 q1 AFF rock which affirms that a pesticide or a pesticidal device is misbranded F quote its labeling bears in a statement design of graphical representation which is false or misleading in any particular manner so there's a number of characteristics that this encompasses these range from safety related claims effectiveness or efficacy statements statements regarding endorsement by federal agency true statements that could be used in a misleading manner as I just showed you in the previous slide or they don't excuse me they don't want you to show comparisons to other products right so comparing other products to your own even if these are comparative safety claims so both the EPA and the states had the authority to enforce this and the fifth row violations cost about twenty seven thousand dollars per shipment so as you can imagine this can get pretty expensive but most companies should be equally concerned about the FTC because the FTC does have the authority to police anti-competitive and unfair and deceptive acts or practices let me explain to you why currently companies are policing for the large part of policing each other they literally turn the competitors into the FTC so this can cost companies a lot of money as well in fines and it also runs the risk of having your products pulled from the market so how do companies go about substantiating product claims for pesticide devices now how do claims are substantiated with data and the data that you need is dictated by the claim maybe you want to make for example a safety claim would require an exposure or risk assessment an efficacy claim would require the development of a study protocol or following some predetermined approved method and that that study would then be executed in a microbiology laboratory so many companies don't have their own scientists on staff you can always hire someone to help you understand what your data requirements are and then develop a test protocol to produce that data so it's really important to be smart a study design and methodology before testing is performed you should define the parameters of the testing and this includes both your positive and your negative controls you'll want to ensure the appropriate quantitative data will be produced as it's important to know how that data is going to be analyzed in advance of the test because statistical methods that you intend to use will also have data requirements and just a quick example a chi-square test that uses frequency data a t-test uses quantitative data and of course the statistical test that you intend to use ties right back to the claim that you want to make so the point here is to do your homework before you spend the money to have the tests run in the lab know when it's all said and done the results can then be submitted to the EPA before review now the EPA not the FTC conducts the data review for design for these devices making pesticidal claims what's interesting is that many companies and many that I've worked with have already performed advocacy studies of their pesticidal devices in order to prove the efficacy to their customers to their clients so all they now have to do is to take that data and submit it to the EPA for review under the prea fika a 521 so the EPA will take about four months and 21 days to complete their their review and they do charge a fee for this it's four thousand nine hundred and sixty three dollars that does sound like a lot but there's a lot less than the headache and cost of being under FTC scrutiny so it's worth it despite the fact that these review categories have been under have been established under prea for what's interesting is that very few of any submissions have been made to the EPA and this could be due in large part companies being nervous about receiving EPA guidance that might be unfavorable to them however enforcement is fairly low for pesticidal devices so companies really need to make a just a business decision they apparently seem to want to take the business risk and sell their product without EPA confirmation which is business decision I'd like to share with you a couple of examples of how companies have tested their devices in order to support their claims so this is an example where the manufacturer produces a device that uses UVC and ozone to disinfect the soles of shoes their target market is hospitals in health care now their sales team found that most of their customers wanted evidence or data that the device actually worked and that it killed in certain microorganisms that were relevant to them they wanted independent test data so in this case a comprehensive laboratory test was devised that used human volunteers in the lab where they were able to simulate typical body weights in Shu materials and actual use and eight different microorganisms that were significant to the healthcare environment or tested but compared to controls the results showed a greater than 3 log reduction demonstrated for seven out of the eight microorganisms in fact there were the end result was greater than 4 log reductions for our Cibola a couple of those microorganisms so it was at least a three log reduction or better which for UVC was pretty impressive so a manuscript of this study had been drafted and it's submitted for publication to the American Journal of infection control where it is currently under review the point is not only will this publication be great for the company from a marketing and BD perspective but the results can also be submitted to EPA for validation in this next example the manufacturer wanted to show that they could effectively disinfect bedpans which are commonly contaminated with c difficile and other enteric pathogens in hospitals and the difficulty of testing this in the field includes the inconsistency of potential contamination from one bedpan to the next along with it's difficult to confirm that the bedpan has contamination that you're looking for specific say specific to seed itself in the first place so again a comprehensive study was developed and executed in a lab which required in this case installing the washer disinfect er in the lab for testing real use conditions were attained by contaminating the bedpans using simulated soil that was inoculated with the C difficile or ecoli and the the results were a 3 log reduction for C difficile and a greater than 4 log reduction for e.coli this these results were drafted submitted peer-reviewed and have recently been published in the American Journal of infection control and there's the reference there at the bottom of the slide if you're interested so again not only will this publication be great for that company from a beedi perspective the results can be used to submit to EPA for validation those are pretty high level overview of studies of pesticidal devices and how the results can be used to achieve multiple goals now there's another really good reasons why manufacturers should ensure that their pesticidal device claims are validated not only will you have EPA validated claims data to present the FTC should they come knocking at your door but now you also have evidence-based science to present to your customers into your stakeholders and since builds brand trust I've seen a number of cases where the data actually told a positive story that the manufacturer didn't even think of before right which then gave them a new edge in that market and to pursue so this data can be turned into communication pieces for marketing business development teams allowing you the assurance for proper advertising and overall positioning in the market with the right data in hand companies can arm themselves with evidence-based science to confidently communicate a product's unique benefits and value while site aeneas lis satisfying that regulatory need so it's a win-win two birds with one stone now there are a few points key takeaways from today's webinar that I do hope that we've communicated to you first it's really important to understand that yes there are regulatory requirements that are associated to pesticidal devices and unfortunately pesticidal devices do not have to meet the same level of scrutiny and rigor that's required of a chemical disinfectant and as a result it has become somewhat of a wild wild west out there with respect to product claims associated to those devices and there are some challenges for example it isn't always intuitive to know if your device falls under the jurisdiction of EPA or FDA or both is that a pesticidal device or not but really the one of the biggest challenges that I see is understanding how to go about substantiating that claim this is largely because for the most part widely accepted standardized test methods and protocols don't really exist for the variety of device types that are out there and with we have a lot of new novel technologies that continue to be developed so efficacy protocol development can be a bit of a daunting task for most people but there are places you can go to for help with that and lastly it's important to recognize that the opportunities are bigger than satisfying the regulatory obligation it provides confidence and marketing MBD efforts and it helps you build brand trust in many cases these studies help companies demonstrate leadership in their space so I strongly encourage validation I have pesticidal devices and with that I will hand it over to back to Erin thank you yes thank you Chris and thank you Abigail for this very informative presentation I think I think there's often a misconception that sense devices don't require federal registration that they're not regulated and clearly based on this presentation we know that's not the case I suppose you know given the current pandemic and the proliferation of devices entering the market to mitigate Public Health pathogens claims substantiation is more important now than ever and Chris I appreciate your comment about soften competitors that turn each other in but I would caution companies to make sure your own house is in order before you turn in a competitor because EPA does have a practice of looking at the company that's raising the complaint as well as the company making the potential false claims I guess that's the saying goes people in glass houses shouldn't throw stones so TSG can help and have been helping companies for many years with compliance issues associated with pesticide devices as proof mentioned there's a lot of new novel products being developed all the time we can help sort out whether the product does in fact a device or a pesticide we also assist companies with establishment obtaining establishment numbers state registrations and as Kurtz has indicated we can help with substantiating their efficacy claims so I'd like to now address some of the questions that we've been that people have been sending let's see let's go to the first question is okay so this presentation is on the US what about Canada what's required in Canada so a wonderful question on Canadian federal registration is required under the pest management regulatory agency or PMR a I will need to confirm additional details with my Canadian colleagues but federal registration is required I don't believe that registration is required in the okay another question my device contains several components from various countries and it's assembled in one location and then labeled in another location do I need to have an EPA establishment number for each location involved in the entire process a-b it's a very common question and I'm happy to address this because there's a lot of confusion here the number on the label of the device is the establishment number for the last facility to produce the device now production does include actual manufacturing as well as packaging repackaging labeling and relabeling so you if you produce your device in multiple facilities each of those facilities does need to be an EPA registered establishment but you only need to have the establishment of the last facility to produce the product have their number printed on the label both facilities must report production this is not redundant EPA's very common and must file annual estaphan report okay let's see we have another question about the CPA work closely with PMR a stop for four devices no the two agencies do not generally talk to each other with regard to pesticide devices or even specifies as much as they say that they do so you know so this this is a question that came up a few times actually and I'm going to try to summarize multiple questions into one question so if the device says for example on it I think this one would be geared towards Chris so if a device says sanitizer for example does that mean it it needs to meet the same performance standard as a chemical sanitizer meaning does EPA apply the same efficacy performance criteria to devices of chemical products thank you for that question it's a good question EPA will have not established a performance criteria for devices we we do advise companies to try to establish that same performance standard as their chemical counterparts pretty much no they haven't established their four devices specifically and that's and that's one of the key components of that study to design on the front end is understanding what become daily requirements you're trying to achieve and what we do to achieve okay great you're trying to we have a lot of questions I'm hoping to get through as many as we can here all right Mike my device is manufactured outside the US does my establishment need to be registered and generally how long does it take to get an EPA establishment number yes your your establishment does require registration whether it is located at foreign or domestic the process to register the establishment begins with first obtaining a company number for the company that operates owns and operates the facility that can be submitted via email on that process does not have a stipulated response time from EPA but we typically see turnaround and about as little as about two weeks once you have your company number and you can then file the paperwork to register your establishment if the facility is located within the US then that registration is filed with the regional office in the region where the establishment or your headquarters are located if your assignment is located not of the United States then you will file that registration with EPA headquarters the process again does not have you stipulated of time from EPA it does vary typically the regional offices are a bit quicker than headquarters and the time of year is also critical the sava tient reports are due March every year for production the previous year and so the response time is typically cold in the spring but generally I would say registration for your establishment attendee process in about thirty to sixty days perhaps ninety for a foreign establishment submitted in March okay another weather question sort of crosses the line of FDA versus ETA so if the UV device is used in for medical equipment surfaces does it require a 510 K in FDA 510k yes yes this is John you're going to be used on medical devices and you will require a 510 K we submitted with FDA okay let's see I sell a water cooler that has UV component I don't make any claims on the unit and it's NSF certified do I need to get an ETA establishment number and comply with pesticide device requirements requirements for pesticides devices where the pesticide divided okay how do I know which bugs I need to test against whatever bugs are going to be claiming on your label so Chris do you want to talk more about that yeah yeah you know like Abby said whatever but the bug you want to claim on your label is definitely the microorganism you want to test against sometime you know so really it's it's where you're gonna use your product and how is it going to be applied so there are specific microorganisms that would be important and speak to your it's if it's health care if you want to say like an example I gave and that it killed Sita well then you would want to include c-diff as a as a microorganism so since there are not protocol specific for device for devices necessarily you know but what we really wanted you to look at the market that you want that your your and enter and then find the microorganisms that speak to that particular market okay so this has to do with efficacy as well let's see did the space use EPA 810 guidelines when reviewing efficacy data submitted during state registration a be sure so the guidelines for those of you who are not familiar are the efficacy performance guidelines the BCA has established there are different guidelines for hard surface sanitizers and disinfectants for laundry sanitization for water treatment etc these are the guidelines that are followed by will say the standard pesticide applicant's pesticide devices are not required to follow the 810 guidelines those guidelines were written with chemical pesticides in mind not pesticide devices although they may be is a tool for you to follow when you're trying to make those claims as we mentioned ETA and the states don't really have a performance standard for terms like disinfectant and sanitizer so the 810 guidelines are a good place to look when you're trying to figure out how to closely mimic what EPA would be looking for for a chemical pesticide to make those claims so this is a related question because there's no performance criteria for devices the EPA regulates device claims such as sterilization versus disinfection versus decontamination no it's a short answer again the agency has not defined any of those terms as they are related to pesticide devices for those familiar with chemical pesticides you'll understand if those terms are very very tightly regulated with specific organism the specific specific contact time and many other testing requirements those standards are not applied to pesticides devices I do caution companies when they are looking at making those types of claims on homicide device to be aware of that you know you must have data on file to support your claims and some of those claims especially in certain new sites can carry quite a bit of weight so if I was considering a claim like that I would want to make sure I had substantiative my data substantiate my claim with data excuse me and I may even consider going to ETA and having me see a review my data to get back sure okay this I'm not entirely I think I'm gonna take that question I'm not sure I entirely understand the question but what a chemical used in barrier packaging be considered a device I think the answer would be no it would not be considered a device okay let's see another question has to do with air filters and air purifiers so two related questions one is our air purifiers that claim to eliminate harmful bacteria considered a pesticidal device as long as they're relying on purely physical means this is an air purifier that relies on a filter or o'the ozone unit or an ionizer then then yes that would be a pesticidal device but if that air unit is relying on some kind of clinical substance to contribute to the efficacy then that would be a pesticide but what about the replacement filter that goes into a air purifier would that require an EPA establishment number so the replacement filter and again this is for a device as long of that filter is not treated with any chemicals no the filter by itself is not a pasta final device because it can't work without these that without the machine in which that filter goes into so that would not require that not require device labeling on that replace that part okay what about is there an exemption for monitoring tracks the monitoring devices are event but you mentioned track so I want to discuss that track monitoring right there events track if there again they're they're purely physicals and those are also exempt but if the tracks include pheromones or other chemicals to say attract insects or to track artistic trap or mitigate or do anything against any organisms then those are do require registration as okay all right this has to do with efficacy testing I'm assuming do claims need to be made using GLP sure the data to be generated under GLP and verified labs I think absolutely so back to the fact that there is no performance standard so I would recommend using a GLP facility I do think that provides weight to your data again some assurance and the validity of those claims but EPA does not have a requirement and the states do not have a GLP requirement for data some of these have to do some other questions have to do with very specific products in terms of chlorine generators on site generators which get to be a little bit more complicated do you want to just speak briefly about precursor chemicals and on site generators absolutely so this is a very complex area I'll start by saying and one where there is not a whole lot written down by EPA so the guidance that we do have is based on experiences with EPA as well as some you know kind of ever-changing registration of some of these products so in general the EPA does if Castle is sold with a device or is sold with language that it is for use with a specific device to have a specific type of final effect then that chemical does require registration as a pesticide with EPA that would be something like if I wanted to sell a specific salt mixture for use with a specific generator then my salt would require registration with EPA the pesticide and my generator would require a regulation as a good one now if I'm not selling a particular chemical for use for the particular device if I am instead selling a device and telling you my customer to go find whatever salt you want I don't care go get a morton salt and pour it in then that salt is not being sold with the purpose of being used as a device it's sold as salt and so it's not going to require registration as a pesticide and again how you sell these precursor chemicals with your device makes a very big difference and this is something I actually was involved with a discussion about two weeks ago with the state of Indiana about a testified product that was registered and is for use with the generator and the question is actually whether or not the generator was a device or an applicator because they weren't sold together and in that case Indiana had to determine that the if that applicator was it could make that that generator for lack of a better term was an applicator so claims are critical how you package it is critical and they're these details or something you definitely want to two parts apart before you get into that so I think we're going to be closing out here appreciate your questions I do realize that we haven't answered all the questions and I'm sorry but please reach out to TSG directly we'd be happy to continue to answer those questions I think you know I wanted to thank everybody for participating we would learn of the questions that we got actually several times is will this presentation be available by email and absolutely and wait there's more we actually prepared a paper on this topic that is hot off the presses and we will send that all to you the paper as well as a recording of this presentation and as well as the slide deck so thank you all for participating I hope you have a great day
Show moreFrequently asked questions
What is the definition of an electronic signature according to the ESIGN Act?
How do I eSign scanned documents?
What makes an electronic signature legally binding?
Get more for esigning substantiation made easy
- Print electronically sign Newborn Photography Contract Template
- Prove electronically signed Indenture
- Endorse digi-sign Summer Chess Camp Event
- Authorize signature service Summer Camp Parental Consent
- Anneal mark Speaking Engagement Proposal Template
- Justify esign Concession Agreement Template
- Try countersign Operating Agreement
- Add Retention Agreement initial
- Send Simple Sales Proposal Template signature
- Fax Baptism Invitation email signature
- Seal Summer Camp Emergency Contact digital signature
- Password Social Media Marketing Proposal Template electronically signed
- Pass Affidavit of Service byline
- Renew Boarding and Daycare Contract esign
- Test Short Medical History signature block
- Require Non-Disclosure Agreement Template signature service
- Comment bystander digi-sign
- Boost proof signed
- Compel petitioner mark
- Void Book Publishing Contract Template template electronically sign
- Adopt Hedging Agreement template countersignature
- Vouch Translation Quote template digital signature
- Establish Work for Hire Agreement template signed
- Clear Startup Business Plan Template template digi-sign
- Complete Travel Plan template esign
- Force Acknowledgement Letter Template template digital sign
- Permit Rent Receipt template initial
- Customize Affidavit of Title template signature