Propose Electronically Signing Request with airSlate SignNow
Get the powerful eSignature features you need from the company you trust
Choose the pro platform created for professionals
Configure eSignature API with ease
Work better together
Propose electronically signing request, within a few minutes
Cut the closing time
Maintain sensitive data safe
See airSlate SignNow eSignatures in action
airSlate SignNow solutions for better efficiency
Our user reviews speak for themselves






Why choose airSlate SignNow
-
Free 7-day trial. Choose the plan you need and try it risk-free.
-
Honest pricing for full-featured plans. airSlate SignNow offers subscription plans with no overages or hidden fees at renewal.
-
Enterprise-grade security. airSlate SignNow helps you comply with global security standards.

Your step-by-step guide — propose electronically signing request
Employing airSlate SignNow’s eSignature any business can enhance signature workflows and sign online in real-time, giving an improved experience to clients and employees. propose electronically signing Request in a few simple steps. Our handheld mobile apps make operating on the run feasible, even while off the internet! Sign documents from any place in the world and make deals faster.
Keep to the walk-through instruction to propose electronically signing Request:
- Log on to your airSlate SignNow account.
- Find your needed form within your folders or upload a new one.
- Open up the template and make edits using the Tools list.
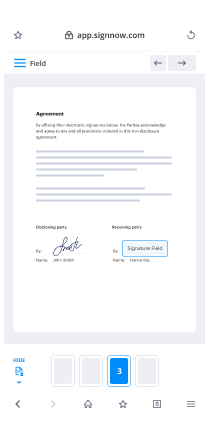
- Place fillable boxes, add text and eSign it.
- List multiple signers by emails configure the signing order.
- Indicate which users will get an signed version.
- Use Advanced Options to reduce access to the document and set an expiration date.
- Click on Save and Close when finished.
Additionally, there are more innovative capabilities available to propose electronically signing Request. Add users to your shared workspace, browse teams, and track collaboration. Numerous customers all over the US and Europe concur that a system that brings everything together in a single holistic workspace, is the thing that enterprises need to keep workflows performing effortlessly. The airSlate SignNow REST API allows you to embed eSignatures into your application, internet site, CRM or cloud. Check out airSlate SignNow and get faster, smoother and overall more effective eSignature workflows!
How it works
airSlate SignNow features that users love
See exceptional results propose electronically signing Request with airSlate SignNow
Get legally-binding signatures now!
FAQs
-
How do you create an electronic signature?
Suggested clip How to Create Electronic and Digital Signature and Sign PDF and ...YouTubeStart of suggested clipEnd of suggested clip How to Create Electronic and Digital Signature and Sign PDF and ... -
Can documents sign online?
Android: Use airSlate SignNow Fill & Sign Android doesn't come with a built-in app that can do this. ... After installing the app, you can open PDF documents in the app and tap the signature button to sign them. You can then share the signed document with another app by tapping the \u201cShare\u201d button. -
Is a signed bid a contract?
A bid does not form a contract. In order for there to be a valid contract it has to be legal, there needs to be an offer, an acceptance of the offer and legal bargained for consideration. A bid is an offer; if you revoke your offer before the party accepts it a contract was never formed. -
How do I get contracts signed online?
Register for a free trial at airSlate SignNow, and then log in. Upload the contract from your computer or from a file-sharing site (like Box, Dropbox, Google Drive, or OneDrive). Add the names and email addresses of your contract signers. -
How do you format a program proposal?
Plan the program. Sketch your problem or point of improvement. Sketch your proposed solution. Define your target audience. Write all the information in detail. Include who the proposal will affect. Draft the proposed solution to the problem. -
How do I digitally sign a document?
Suggested clip How to Digitally Sign a document with airSlate SignNow Reader | Acrobat X ...YouTubeStart of suggested clipEnd of suggested clip How to Digitally Sign a document with airSlate SignNow Reader | Acrobat X ... -
What is proposal and its types?
A response to a Request for Proposal (RFP) is one type of solicited proposal. Most RFP's have a stated deadline and are one-time solicitations for specific needs of the sponsor, not expected to recur. The proposed project must respond to the specific work statement in the Request for Proposal. -
Is a proposal an agreement?
A proposal is an agreement being submitted in anticipation of being signed, or otherwise legally accepted. If everything is in place, once that proposal has been accepted, it should become a legally binding contract on both parties. -
How do you create an electronic signature in Word?
Place the cursor in your Word document where you want to insert a signature. Click the Insert tab. Select Signature Line. A menu will appear. Fill out the required fields. Select OK. -
Is a proposal considered a contract?
Proposals. ... A proposal can turn into a legally-binding contract, but the language of the contract doesn't have to read like a proposal. A proposal becomes a legally binding contract if you've instructed your client to abide by the terms of the proposal, sign it, date it, and send you funds. -
How do I create a digital signature?
Click the link. ... Agree to electronic signing. ... Click each tag and follow the instructions to add your digital signature. Verify your identity and follow the instructions to add your digital signature. -
What is a proposal in legal terms?
PROPOSAL. An offer for consideration or acceptance.It is a general rule that a proposal offered to another for acceptance may be withdrawn at any time before it is accepted, provided that notice of the withdrawal be given to the party to whom it was made. ... PROPOSITION - An offer to do something. -
How do I create a digital signature in PDF?
Open the PDF file in airSlate SignNow Reader. Click on Fill & Sign in the Tools pane on the right. Click Sign, and then select Add Signature. A popup will open, giving you three options\u2014Type, Draw, and Image. Once you're done, click the Apply button. Drag, resize and position the signature inside your PDF file. -
How can I make my signature?
Suggested clip How to design your own amazing signature - YouTubeYouTubeStart of suggested clipEnd of suggested clip How to design your own amazing signature - YouTube -
How do I insert a handwritten signature in Word?
Sign your name on a white, unlined piece of airSlate SignNow. Scan the signature and save it as a bmp, . ... Start Word. Go to the Insert tab and select Pictures. Navigate to the signature file and select Insert. Select the image and activate the Picture Tools tab.
What active users are saying — propose electronically signing request
Related searches to propose electronically signing Request with airSlate airSlate SignNow
Propose electronically signing request
well good afternoon and good morning uh once again glad you could make it to beyond compliance for december 2020 uh where we're covering some regulatory items coming soon compliments of the fda's new traceability rule and we've got seven key points everyone should be aware of right now um i'm glad you could could be on on the the line with us my name is aaron bolshawn the vp of marketing for safety chain and the moderator for beyond compliance and for those of you that don't know or may be new to it beyond compliance is an ongoing monthly industry update for food beverage cpg and process manufacturers specifically the fine folks in the line you know facility operators qa safety compliance professionals even the lab we partner with leaders across different sectors from like labs equipment manufacturers consultants regulators lots more really just with one goal which is to bring insights around the market that shape the way we grow our businesses deliver quality products to our customers and also safeguard our brands we do try and give you some things every time that you can take back to the plant with you to improve production compliance and quality with those that's our goal today as well safety chain sponsors the webinar series and uh safety chain is the number one plant management platform that improves yield maximizes productivity and ensures compliance uh specifically for usda fda all these good regulations right for food beverage and cpg companies um we're the only complete solution for for production uh you know think about oee spc food quality uh and safety the qms side of the house and even supplier compliance and beyond so all right with that we are scheduled for about an hour uh as we often do with these webinars we'll take some time at the end for some questions we can't hear you if you talk we're not going to hear you but you will hear us we do want to hear from you so use the webinar console to submit any questions uh throughout the presentation we'll save some time like i said at the end to cover that and i believe we'll have a lot of good questions as we have uh time to get to them so if anyone has any audio issues just uh try pick up a phone call call and usually that resolves it and then everybody will get everyone on the line anyone that registered for this will actually get a recording we'll get the um the presentation deck so you'll be able to take this back into your facilities and share as you wish yourself with that i would like to introduce our panel so uh we got a powerhouse today so shamanic shriek is a solutions architect here at safety chain and uh she's brilliant she's got over 10 years of experience as a food safety expert and auditor and quality specialist a couple of degrees more than i have biochemistry molecular biology so the very science that connects the uh food into our systems is she's an expert and she's worked at treehouse cargill csm bakery and i was also a microbiologist at miller coors um shamanique welcome thanks for being here thank you all right and then eric edmonds lots of really interesting and way i say this a lot uh uh uh eric the the venn diagram that you you expect of your experience is really unique um got a combination of legal regulatory and food industry expert you bring that to the atchison group and you also join us on fisma fridays certified hassip registered environmental health specialist um you've got a preventative controls qualified individual here with us today for fisma and also produce safety rule trainer so the list keeps going on and on uh eric thanks for joining us once again thanks for having me great to be here yeah i think so both of you guys are going to make the nice list this year with all the generous dedication of time you're giving uh to beyond compliance and then eric i know we've been talking about the stuff that's on fisma friday as well so i just can't thank you enough both for for joining and we do have a lot of ground to cover today um but i i just wanted to start out very quickly for for all the viewers just a couple of things um we want to give you today and want to you to be able to take back to the plants one is you know just an understanding of the new fda traceability guidelines right what are the kdes who's on the ftls all that good stuff but two we wanted to take an approach here that was a visual approach we wanted to give everyone a view of what it could look like in in a digital perspective because some of the uh requirements right so you'll see the safety chain platform in front of you but our goal here is not to sell you software or consulting services we we just wanted to be visual in the way we represented and visual in the way we showed some of the records the fda will be asking for and shamanic will be showing that um a little bit later so with that um eric why don't you take us off uh give us here's the top seven here's what we're gonna go through if you if you kick us off uh and i'm gonna be controlling the screens here for us and just let me know when you wanna uh need a passport okay sounds great so yeah a lot of ground to cover like you said aaron so today we're gonna focus on the real key factors the the new terms from the food traceability rule as it's proposed so we'll cover really what fda is calling the food traceability list which is that list of high-risk foods that are going to require some additional traceability records at different spots along the supply chain and that's where you get we're going to get into the the new terms the fda's put out there for this rule the ctes and kdes which are critical tracking events and key data elements along with supply chain that the rules calling out is new new requirements and uh which of those apply to different people along the supply chain so the the real critical tracking events that fda's lined out is just points along the supply chain where these items are grown received shipped transformed or created uh whether through repacking or kind of processing um as well as some kind of more i guess less requirements or lack of requirements when kill steps are applied along the supply chain through a validated kill step during processing um as well as the record management requirements and as aaron said shamany is going to touch on really what it looks what it could look like in a an electronic program for managing all these records and and keeping that all uh there so you can supply the information to fda as needed upon request and we'll we'll touch a little bit about kind of the comment period and the overall timelines and deadlines for when fda plans to be enforcing and um really when the rubber hits the road with the rules and a few years after or a couple years after after the final order was proposed so we'll we'll touch on all of these seven seven items today so we can jump to the actual list and kind of just provide an overview so fda's been working on this for quite a few years it's part of fisma really so 2010 when uh president barack obama signed fisma this is one of those aspects that fda's been working on ever since and this is just finally getting out to uh kind of the the real actual proposed rule they've been real busy with all the other kind of seven pillar rules of fisma um but this has been an ongoing process since right around the time fisma has passed so as you look at the list you'll see it's a lot of the usual suspects that you've seen in the news for recalls the foodborne illness outbreaks in the past 10 to 20 years especially um so it's kind of what you would expect to see fda identifying as what would be termed as a higher risk food that has some additional traceability requirements so they really created the list by looking into the history of all the foods of each particular food so they looked into has it been involved in a recall recall or a food worn out illness outbreak and they also looked into kind of the points along the supply chain where contamination was likely to occur as well as as well as where it may be controlled through something like the kill step um and just really looked into really focusing on microbiological and chemical hazards within within this uh list um so most of them are kind of whole items but some of them are further processed items like they're ready to eat deli salads that you that you see there at the bottom of the list but this is the proposed list so there is a chance that through the comment period it'll change slightly um i don't don't imagine it'll change drastically but uh there definitely be some comments from industry and the public that that may push fda to a flight revision and of course fda leaves the opportunity down the road that they may be adding new items to the list if new products their items are associated with foodborne illness outbreaks or that sort of thing so the key terms that we're looking at here uh critical tracking events is really the steps along the supply chain the fda feels are key to having information available so when an item is grown shipped processed received or created and those are the the points along the supply chain where records are going to be help fda with both their trace forward and trace back um investigations when especially when there's a recall or a foodborne illness and the key data element is the information at each of these critical tracking events that is going to help fda facilitate uh an actual traceback or trace forward exercise so they really use this on some based on a pilot program that was conducted by ift uh since fisma was passed that identified some some weaknesses in the kind of traceability of the supply chain of the united states and what they really kind of keyed in on is the things that were hampering investigations where first not all aspects of the supply chain were really covered by what fda typically has as their traceability requirements as the up one up one down type of requirement so they said farms and restaurants or retail locations are largely kind of exempt from that which makes it really difficult to even start the investigation typically because the retail location or restaurant is going to be the one that the person who becomes ill directly counts um saying this is what i ate and this is where i ate it this is how i got sick um but the fda would go and not have all that information available in all senses they do recognize that a lot of companies out there have put in great traceability systems but even when they have when you go along the supply chain from company to company the data wasn't necessarily lining up so these key data elements you're going to kind of really say this is the information we have and so it'll be present each step along the supply chain so fda will be able to go from company to company to kind of track this information to really where it may be sourced or where where the contamination may have happened um and part of the rule um and what the statute part section 204 of fisma really did is kind of said fda can't necessarily require companies to really create a brand new program or contain the information along the entire supply chain but they should have this information already in some of their records so reference records is really a long list of potential records that a company may have that has this information so anything from a processing record when uh something's being transformed or created to just a bill of lading or a shipment record um and that's something that uh you'll have the information there um and the really one kind of an electronic system is going to become really useful is organizing that information and providing to fda when when they request certain things um the traceability lock code and traceability product description really are what most of us are familiar with traceability lot code using kind of an alphanumeric number or descriptor to identify a specific production code or packing uh packing lot to um kind of create bookends of different production dates and the traceability product destruction is literally that a description of the product so we have listed there that there's a difference between single ingredient and multiple ingredient products and the real difference there is so the general things are going to be the same for both that this is the amount of the product this is how it's packed whether it's a clam shell or a case or a pallet um things like that but the single ingredients actually will call out the specific single ingredient or commodity that's there while multiple ingredient products will kind of include the overall product description uh whether it's a deli salad in a clam shell or a tote type of thing um so that's that's the the real difference there is single ingredients can actually call out a single product by name whereas multiple ingredient products will be more of a description of what the product is let me jump to the next slide thank you and uh generally like i said fda expects most companies to have this type of information already there it's just not going to be necessarily organized in a single location so what the general program overview is going to need to do is list and describe the reference records or the where the information is located and how those reference records are linked so a bill of lading or receipt for receiving an ingredient that may be used and processed into another ingredient that's tied to those batch records and finally kind of the finished product lock codes um as well as a description of where to find that information on their records uh companies will have to refer back to that food traceability list and kind of keep a tracker on all the items that are on the list that they use or produce as part of that and kind of include how they're assigning traceability law codes and how they're describing the product there and then just to kind of deal with that issue that i mentioned about how companies use different terms and different coding systems there needs to be an explanation within the program that fda will be able to understand your data so how you assign a lot code um any abbreviations or special terminology that you use within your program that's not the same as everyone across the country there will have to be a general description of that so fda can look at that overall description and really understand the data that they're receiving from you so we're going to start with the i mean the bare bones of when the food products are originally grown so records for growers are pretty simple and straightforward um and that's that they need to have an actual growing area coordinate listed as part of their production record so fda refers to this really as the gps coordinates that's kind of the width the location at which you would enter the growing field there are some different requirements for sprouts and fish that are on the list so sprouts have some specific things about tracking kind of the person who cleaned the seeds or treated the seeds along the supply chain and farms may end up getting some of those additional records as well if they hire different people to harvest or cool or pack the product um but the the real key for the farmers is are those growing area coordinates um and just like the gps coordinate for a farm or the location where things are grown fishing vessels will be kind of recording the dates that they were at sea and kind of the national marine fisheries program kind of gps coordinator kind of area in which the the seafood was harvested was what they're going to be looking through as the the gps coordinate so um yeah growers are pretty straightforward and um yeah we can move on to the next flight yeah you can kind of tell us a little bit more this is where we want to do a little bit of okay what what does this really look like right so go ahead so many things right so from the point of view of a grower uh like eric said it's pretty straightforward um so a grower in this example would literally just write in their traceability lot quote like starting out and also those growing coordinates um which has typically been something that's missing when it comes to doing a recall especially for like leafy greens and things like that which is why so many times we don't actually know the source of a foodborne illness we just know that a certain amount of romaine lettuce has been contaminated we can't exactly pinpoint the location this specific record is supposed to help with that in the event of a recall so pretty straightforward like eric said having that traceability lock code associated with the growing area coordinates for that specific lock code okay and the next step along the supply chain is really these records for receivers so the records that a receiver is going to have to keep are really similar to every everywhere else along the supply chain that we'll cover later on in the slide but um the records that they're going to have to keep is that location identifier and location descript description for the immediate previous source so that is the just the the gps coordinate of the farm as well as kind of the um kind of the area in which it was done um if that's an international farm um it's going to have an import number that will be tracked as part of these records and the location identifier and location description of where the food receives so that's that's your facility um whether it's a warehouse or a processing facility that just gonna have to be recorded as well as the date and time that you received and then you get into these other aspects that really tie into that product description how much are you receiving how is it packed um what type of packaging is it in um and kind of that traceability product identifier that is associated with those packs um the the next one is kind of the point of context for the traceability lot code generator i'll touch on this a little bit in one of the next slides because there's a certain classification of the receiver that fda is calling the first receiver but in most cases that traceability lock code generator is going to be the farm um but in other instances where there's kind of multiple people at the farm level who are cooling harvesting packing um that first receiver off of the farm may be the one to create that traceability lot code so that's where that could potentially change as well as like i said just what reference records are you using to maintain this information and who brought you the food are the key records that are for those general receivers so on this form like eric was saying for records for first receivers you can record all of the information the traceability lock code the supplier lock code quantity received unit of measure all of that information in this form we can also there's three ways that we could potentially get this information we can do manual entry we can integrate with an erp and that information could be pushed into safety chain so that you don't have to do do double entry and it's accurate and it's a way to house all of that information in one space or you could potentially do scanning of certain items or ingredients as they start to come in or paperwork like that we won't be a traceability lot code generator but we can link those traceability law codes um to the different ctes as it goes through the process so the very first one would be the records for receivers for most people as they start to receive that information in and as eric was saying when you receive in something you have to record your own location the time and everything like that in an electronic system once a record is submitted it's going to be that information is automatically tracked for you it's not something that you would have to do extra to say this is our location um address and the time that it was received once it's submitted it's automatically recorded okay and as i mentioned these first receivers is this unique classification of receivers and it's the first person other than the farm that purchases and takes physical possession of of of a listed food so really the records for the most part are the same as any other receiver but like i said in some of these kind of the way the on-farm process works is there may be multiple parties that handle or deal with the food before it actually gets to an off-farm location so there may be a chance and times in which the farm or one of these other groups don't assign an actual traceability law code and that's when the first receivable will be the actual generator and the originator of food under the rule um but they will have to have some of those other information so generally the farm information the gps coordinate but if someone other than the actual farm is in charge of harvesting cooling or packing the food before it gets to that first off farm location information for all of that is going to be part of their records as well including kind of the date and time that each of those activities took place um and the seafood like i said it's really similar um they may be creating a traceability lock code but the information that they'll be having is the harvest date range that the the ship was at sea and the kind of the gps coordinates or the national marine fisheries uh groups kind of coordinates for kind of the the fishing area where the seafood was caught um instead of the gps gordon of the farm so this is an example of that first receiver cte form we can essentially record the cooling the location identifier for cooling of the food for the packing of the food we can also have a place where you can type in your record reference type and number if it's an invoice if it's a bol if it's a packing list all of those things can be easily referenced on this form so that links that traceability lot code to all of your reference record types so when it comes time if you did have a recall everything is all together in one place with that important information um yeah that's it for this one okay now we get into a little bit of the more actual processing aspects of the rule where ingredients could be coming in and leaving something else so transformation is when you're dealing with a product or an ingredient that is on the food traceability list and converting it to something else and this doesn't have to be a major processing where you're drastically changing the nature of the food or anything it could be as simply as simple as adding a different label or packing into smaller packages so going from from the case of produce down to the clamshell level you're changing kind of the appearance or the way the food's packed in the way a customer would identify it or anyone would identify it along the supply chain so really any change uh of the food that's on the food traceability list into something else is a transformation period whether it's something as minimal as is repacking or re-labeling a food there are different records that are going to be required in the way they that it's described as food traceability list items that are used in transformation or produced through transformation so uh the records are slightly different for those because of what what what you're doing to the product so for those ingredients that are on the list that you're using in transformation um it's those are receiving records that you're going to be keeping so the traceability lock codes as well as the products identifier product description and the quantity you had however when you transform those into something else the records that uh you're going to have to after those foods produce through transformation so the the final product that's going to be leaving your facility is going to be kind of the location identifier of your processing facility or repacking facility and as well as a new traceability product identifier and traceability product description so if you're going from a case of romaine lettuce down to clam shells with remain lettuce or a salad that uses the roommate minus it's going to change that product description to clam shell and a different weight of the actual finished product so the ingredients used is essentially your receiving records but then you also have to have some records about the actual item that you produced as well my guess eric is that we'll probably get some questions revolving around that because that is because there's some separation there uh between in in you know used in or produced through uh that's gotta that's gonna have a little bit of an effect shamanic what's this look like um like eric was saying it looks just like that we can record the finished product traceability lock code as well as the traceability lock code used of the ingredient and we'll also be able to have track the quantity of that traceability lock code that was used in the unit of measure and for reference record types we can get information from a process order for that transformation activity like i said if we can integrate with someone's erp to have this information already pushed into safety chains so it's not something that you're um recording again but if you do not have an erp system that you aren't currently using and you need to have you're going to have a manual process for how you're doing this this would be really helpful to help to link all of those different ctes and keep track of that traceability law code as you go through your process yeah and that's a really good point shamanic where you know the labeling of your form name and this is whether you're creating this as a digital record like you're seeing here in the safety chain platform or if you're doing it still manually if it is on paper please give yourself a break labeled them very specifically foods used in transformation or or through so uh thank you appreciate that and i think the next slide actually just shows like regular uh records for transformers so you can definitely differentiate between the two and make sure that you record all the proper record reference types um and i will show a little bit later how this all kind of comes together at the end when you do have the distinct uh naming conventions for your ctes yeah and one of the things to mention here too is um it's hard to see on the screen but there's a couple of red asterisks next to uh some of the the form fields on the left right and this is something that is pretty interesting like if you you need these things to abide by the the ruling right or the guidelines and so if you need to your operator you have to force the operator to create create that record or create that specific piece of data um whereas you know in in start contrast if you're using a piece of paper somebody can skip over a piece or a section of that paper pretty easily especially if you think about like a new employee or something that's just getting started or something along those lines where a digital record will be able to help you guide the operator through the process as well so okay and jump into the next one uh creators is one that can be a little bit confusing with transformation but so with transformation where you're actually using ingredients that are on the food traceability list a creator these are the records of when you're creating a food that's on the food traceability list from ingredients that are not on the food traceability list so the the real prime example here is going to be the nut butter category that's on the list so peanut butter for example is typically made with not very many ingredients but none of them on the list so you may have uh the actual peanuts salt and sugar or something in there um none of those are on the food traceability list but you so the none of their record requirements are going to be coming into your door but you're creating a product that is on the list so this is when you're going to have to have the kind of records of where you're creating the food and you'll be creating the traceability product identifier as well as the description and the amounts using amounts that you're producing and kind of how they're packaged so these this is going to fall into the arena of kind of those production records and batch records for the product you're producing um for the lock codes which is really similar to what a lot of people are already uh applying i do have a note here that like i said retail establishments are getting pulled into the food traceability list but both for creators and transformation uh of products of a retail establishment if they're selling those products directly to a consumer they will not be required to keep transformation or creation record as as part of their system um because it'll just uh kind of caveat it would be much too difficult for so many real tech retail locations to get those records on a daily basis and create those and so um uh the customer should be able to kind of say well this is where i bought it and then um the company would have their receiving records but not gonna is not to be required to keep the creation or transformation records at the retail level yeah and that makes me think uh eric of the the peanut butter grinder you know the the peanut butter makers in the back of the store where you can kind of do dump in your peanuts and actually grind your your own peanut butter that's something like that so that's that an example of where that retail establishment is just too too overwhelming to think of uh to get through there so what uh shamanic what's the the creator's record look like walk us through this one is fairly straightforward i also use the example of nut butters for the creation event um and this is where that traceability lock code would be generated if it's a creation event um because like eric said it's made from an ingredient that's not on the ftl list so you will you would record the traceability lot code number uh the lock quantity unit of measure and any other record reference types and numbers that could be associated with this creation event um yeah it's pretty straight forward from a forum perspective so uh as we mentioned shipping of these products is one of those key items that fda identified that they need to have their records so the difference in these is we've kind of put in bold all these records and that is what when you ship a food on the food traceability list you're going to have to include this information with the shipment so it's getting to the the next stock along the supply chain so the if it's imported those entry numbers as long as your as well as your product identifiers product descriptions and how much you're actually shipping um the bottom two records are more for internal purposes that don't have to accompany the shipment so those reference records that you used either through your transformation creation or just receipt of one of these items and you are going to be required to kind of record who you're hiring to ship or transport the food for you but that information doesn't have to actually accompany the food not that the receiver is not going to notice who is bringing the food to them so they'll be able to get that information on their own but it doesn't have to be listed on your on your records that you're including with your shipment [Music] and this is an example of the shipping cte form this information typically would be recorded on like your bol a packing list it might come from a purchase order that's being held within your erp system um it just basically houses that information like i said either through manual entry or through integration and that's that's key you know when you start chuck integration shamanic the systems that are you're using to help with the fda traceability and maybe just the other overall compliance or certification systems and programs that you have that's a key point you'd be able to integrate into other things you'll look for apis and things of that nature and you want to more fully automate these things so right because a lot of times this information is just housing different file cabinets receiving like has their source of paperwork that gets put in a file cabinet and then this way if you have an electronic system that like saves train that integrates you'll be able to house all of those things and see the traceability all the way through to make it easier to reference back to the things that you may need to get a hold of um in the event of a recall yeah and this is the the whole point right guys so eric we're going to get to the point of hey when the fda comes to you and says hey we need to see these things there's a turnaround time that's been mentioned in the guidelines and again this is going to be settled through the over over the next many months right but there is going to be a position there's going to be a point at which you have to be able to turn these records around to that organization to that governing body very quickly right so um with that i just want to take a second i'm going to pull everybody here uh eric and chaminae to to ask you very bluntly and and by the way just before i open up the poll just make sure everyone understands no nobody's names are attached to these we're not going to show anybody's names or where you're at on this um but it's just to give us an idea of who's in the room with us who we're talking with and and what we wanted to know is what your confidence level that your facility could easily produce a sortable spreadsheet within 24 hours of all the kdes for specific ingredients used in the prior three months so think about this right you're asked by the fda hey we need these um this data right uh over the last three months to help us with this investigation or whatever they're they're looking for and we need it tomorrow so i'm going to launch this poll it's going to show up on everyone's screen and when i do that please take a moment uh everyone if you will it should be coming up on your screens in just the next couple seconds here when you see that take a second think through there's three options are you confident you know already set up to do this okay with little effort uh you're semi comfortable right so you you can produce this information but it's going to kind of produce a lot of added effort right and then there's this questionable right we're really not ready for this level of compliance where hey i i can i need to turn something around on a dime so um we've got a bunch of people thank you so much for going we've only got 40 voted so um eric where would you if you had to guess um the the percentage of the confident people that are like i'm there we've got it where do you think that'll end up before i show the results no i'm well i'm thinking probably less than 50 just because i mean everyone's used to this 24-hour requirement for fda kind of getting your data or your information or records but typically that involves fda coming to your facility and you can hand them your binder or say oh well let's get the shipping people are receiving people up here and they'll provide all those records and they have them on paper form but it's this kind of unique thing that fda is asking um you know we need this in an electronic sortable form real quick um so it's that format that's a little bit different um i don't doubt that people have the records but actually having them in that format and organizable is the difficult part you're right definitely less than 50 so uh but not bad actually so i think the bulk of everyone definitely they can get there uh it's going to be added effort might be painful it's going to be one of those things where you're going to come in uh to the office to the plant and you're going to have this email and you're going to go oh boy here we go again so um uh and then there's a you know full quarter of us that really just aren't really ready we're using some paper records but we're glad you're still here thank you for being honest and open about that as well um you know that's that's part of uh really being able to uh uh cut you know cope with this in in general so i'm gonna pull back up the slides here just give me two seconds guys um and then we're going to continue with some of those records okay so let me go back to close the poll sorry about this hide results there we go so um next up we've got uh you know the kill steps so there's the whole other sort of uh ball of wax here won't you take us through that a little bit eric yeah and this touches on that aspect when like i said when fda was creating the list they really considered where in the supply chain is contamination likely to occur and where in the supply chain could it be controlled so this kill step is basically a something that will stop the requirements for having these records if you apply a validated kill step to one of these products um the records basically stop there so you would have your receiving records for the product but then you apply the kill stat the records end you're not going to have to keep any shipping records that are required under the rule or anything like that and as well as that the person that you send the product to is not going to have to keep receiving records either because they'll just have information that a validated kill step has been applied so these micro biological risks have been controlled and that's where fd is saying well then the need for this traceability information isn't is great because the risk has been controlled um so they will have to have it validated which i'm sure a lot of companies have been working on their validation studies for kill steps and cooking process since fisma has put that into a as a real significant requirement as part of the preventive controls rule um but it is that validated skill said that basically it says once you apply that validation step subsequent records required under the food traceability list are moved because the uh the risk has been controlled okay and this is where i'm going to touch on that basically that poll question that aaron just had up so the typical provider records as soon as possible but not after uh but within 24 hours is what you're looking at which everyone is used to so that's how fda may be looking into kind of checking in to see if you have a program that's in compliance but this electronic sortable spreadsheet requirement comes at any point when the fda is investigating a recall or a foodborne illness outbreak or anything that causes a potential issue or threat to public health um and what fda is going to do in these sense they're basically gonna they're going to contact the company and say i need all your traceability records for nut butters between the dates of september and october of 2020 let's say and within 24 hours you're going to have to be able to compile all of that information an electronic sortable spreadsheet for the fda that identifies the time range that they've asked for as well as all the food products so a lot of times this will be specific to a single food product that they suspect is part of the foodborne illness outbreak um so it will be somewhat limited but really just having that information in that format is going to be the difficult part so fda can kind of look through it really quickly um and that will be a company that kind of those those other information kind of acronyms and things that they may need to understand the data that you're providing and having an easy access to a system that can do that kind of reporting is uh you know obviously makes life's uh much simpler and charming i can just imagine like this um this request coming in and people scramble to the those the filing cabinets and start pulling stuff out if they haven't already in their processes already transcribed that data into a spreadsheet somewhere but this is another look at how that can work yeah yeah so um one of the good things about using an electronic system is you can leverage programs in order to keep all of that information housed in one place so i basically built out a food the food traceability list and use our programs to um manage this process by item and those forms on the left-hand side are the different form names for the different ctes um and you wouldn't have every one for every product it just depends on what your facility receives in but for the sake of this i just added them to the entire thing and then on the the right hand side here i have um basically that representation of over the course of a week this is how many ctes um were performed and those are the different records associated with them um you can see well kind of it's a little small the different resources whether it was cheeses leafy greens nut butters uh fruits and vegetables that's also available and so that information can be exported or yeah exported to an excel spreadsheet as eric was saying and if you go to the next slide um this is that data for you internally for you to see over the last 30 days how many um records you have by resource so that information that we saw on the previous slide it shows that i had four records for fruits and vegetables cheeses tomato one and then one for the rest of the ingredients that i submitted a form for and if you look at the traceability by ctes i'm also able to see over the last 30 days how many different ctes occurred or how many records were submitted for those individual ctes and then if you look down at the bottom i can also see the fda traceability lot codes and so the first one shows that that lot code has four ctes associated with it and that would be my fruits and vegetables and that's associated with that and if you were to click into that that column that says four it would also tell you the specific cte events associate associated with that particular lot code i just didn't show that completely so this is what i mean when i say that we can help manage those traceability lock codes for you and show how they're all connected as they go throughout the different ctes um and it's very easy for you to see and you can easily set the time frames for the last 30 days 60 days 90 days however you would like to see it and then you can export that data easily into an excel spreadsheet for the fda should they need it so this is an example of how an electronic system can help this process and kind of streamline it for you okay and just to touch on kind of the overall timeline for the rule in closing um so as i said it's a proposed rule and so the fda is accepting comments um if there's something that you're not understanding or you want clarity on or you think is totally wrong um they're they're open to that and you can submit comments to the federal docket um until january 21st of next year um and then fda is going to take all those comments that they receive and evaluate them and kind of go through the rule and all the comments with a fine tooth trying to calm before they release a final rule um which will become effective 60 days after it's published so that timeline we'll see how long it takes obviously with these big rules that affect a huge amount of initiative there's going to be a lot of comments so it's going to take fda a little bit of time to actually do a thorough review of all those so um but you should see that sometime and hopefully in the next year or so um that they release the final rule and then after the final rule all that says effective after 60 days companies will be allowed two years to comply with the rule and with most of the fisma rules you've seen kind of this tiered compliance timeline for different sizes of business but the whole point of this rule is saying um basically everyone needs to be covered at the same time otherwise the the rules were a little bit well i don't want to say useless but not very helpful if not everyone's doing it so there's no tiered uh compliance timeline for enforcement for you depending on company size so everyone's going to have to have that at the same time um there are a lot of exemptions and modified requirements for the rule but they're somewhat limited at the same time so um the only companies that are like completely exempt from this rule or people are going to be really small so if you have less than 25 000 in annual sales as a food processor you're going to be exempt or you're a farm with less than 25 000 in annual sales you could be exempt but that's a pretty small category of the food industry as a whole um and then there's some kind of partial or modified requirements for some of these people um that uh we're not gonna go into in a lot of detail here but there's something that's worth looking at to see how it may affect uh the overall program and so we're going to wrap up you guys uh we're getting to the end of the the hour and i want to encourage anyone if any questions uh submit them now i see a lot of good questions about some cheeses and we're going to get some other good stuff from vicki and deborah and so forth but right now i just wanted to take a step back okay i'm we're in the business of helping uh food and beverage and cpg manufacturers just get their work done better easier and live better lives so but where to start i don't think that generally speaking the the facilities you know the c-suite the folks that are going to be building budgets and so forth for 2021 they're not going to buy traceability okay if you are looking for a solution uh here's a couple of points though avoid the point solutions uh if you'll in the long run you'll have um one thing that does one thing really well that isn't flexible enough to meet future um regulatory complexities and that's what is a absolute for sure the complexities in regulations will continue to to grow so um and when you start to discuss these things you're going to inevitably have an roi discussion okay what's the return of an investment um to us right i would avoid trying to talk about just traceability specifically but talk more broadly about audits and overall risks right the full regulation gamut and and the certification side of the house and then even auditing suppliers i mean that's critical in this whole thing anyway so so um and then the last thing i say is just get some help understand your options um we do have an executive guide to plant management software that's out it does go through very specifically the roi of something like this and it breaks it down piece by piece and you will actually see three areas in that roi guide about audits and overall risk regulations and certifications auditing suppliers if you do need some place to go and to get this discussion started for your facility we encourage you to take a take that and read that so um with that we are going to jump into some questions guys so i'm going to um lob them over and i'm just going to pick on you shamanic or you eric a little bit here and there um the first one and i had the same question and i you know eric i've been meaning to ask you this offline but how is the the hard cheeses defined is there like uh vicky asks is there a maximum moisture specified for hard cheeses or how does that look um so it's actually soft cheeses is what's covered so the the cheeses other than hard cheeses is what's on the food traceability list and the way that fda defines that is it it doesn't call out moisture content or anything that says all soft ripened or semi-soft cheeses and fresh sausages that are made with pasteurized or unpasteurized milk so soft ripened semi-soft um cheese is the general category but it doesn't go into specific um kind of water content requirements and things like that got it thank you that's uh that's good to know because in my mind i'm like yeah exactly some of the cheeses i've had you know over thanksgiving break were pretty hard but i didn't know that would be covered all right so uh excuse me laura laura can we uh ask based on this rule can we ask vendors uh to produce uh send us a lot information with delivery notes or the po because some products like produce do not have lot codes you definitely can ask so yeah that's kind of the whole idea is um the fresh produce and that's why where they kind of say the farm may not actually assign a lot code or the grouping of farms harvesters coolers packers but the first person off farm that receives that product it is required that they do create a traceability lot code so that shouldn't be an issue anymore they should be creating it's required that they do create a traceability lock code for for fresh produce um or lettuce um when it's first received in a non-farm location great another cheese question uh i have this feeling it could be some folks in wisconsin not sure but uh so if i purchase cheese products that have already been packaged and purchased from the manufacturer or distributor will these products come in with the traceability product identifier identifier seasoning and product description so after the dust settles will that happen yeah it really should so um if the manufacturer is creating and packaging it like finished consumer product they would have created that traceability law code and product identifier and that is part of the shipping record that they will be required to send along with any shipment that they're sending to you very good very good uh got an interesting input this isn't a question xiaomi but you'll like this um so rob uh on the phone on the uh on the webinar said that he didn't respond to the poll that we produced as it doesn't apply to me as an fda investigator in my experience however i would agree that less than 50 percent of firms are confident in producing documentation just an observation i appreciate that robert thank you for that that input that does help us and again you know about that 24 hour turnaround time frame for records marcus asks there was a mention of an email altering the plant uh of the of the need is there a definition of how this uh alt this would come you know is it an email is it a phone call official letters from the fda i'd love to ask rox but uh shamany how do you usually see these come into the plant like when you get notified from the fda what's that look like is it a letter is it an email it's a call or sorry say that again the fda is going to come yeah if the fda is going to come knocking right how did they not show up [Laughter] rob's still out there laughing he's like i don't call first no [Laughter] yeah i think ultimately yeah they just kind of say hi we're here no marcus that's a good question generally speaking um you know i don't think that there is a prescribed way that the fda does reach out to you i was just giving that as an example of hey when you do get to that point and fda is coming around um you gotta be ready so um deborah i've got a question so um salad leafy green products do not get a validated kill step so we will have to trace all the way to distribution question mark so i think that's a yes or no is with the salad leafy green product there's no kill stuff so where you were you tracing through exactly so um it's each step along the supply chain is going to have those records and i one of my favorite terms always nobody cooks their lettuce um so yeah it's never going to have a kill set so those leafy greens are going to be the ones that you're not going to be have kind of the exit through a kill step at any point that i'm aware of um that it will have that the records along all the way through distribution to the retail location it all right and we are um uh and kind of and end of the time here we've got a few more people i appreciate all the um questions we weren't able to get to uh every them uh everyone rob again from the fda did say yes true we just show up [Laughter] so rob thanks we're gonna i'm gonna reach out to rob we might have him on beyond compliance we'd love to to get some insight um you know it's often uh uh a really good point of view to get from the fda i know there's a lot of hoops that rob would have to jump through to be able to do something like that but i might reach out to you rob anyway so hey listen there's a lot more uh we could discuss but we've got a lot more resources for you online uh safetychain.com go to um theatersongroup.com as well they've got a lot of great stuff and join us um again uh every month we also do fisma friday uh go sign up go go engage with us online um uh on linkedin and facebook and and and twitter um we'll keep you we'll keep you in the know and we appreciate everyone's time today shamanic and eric once again fantastic work thank you for for for joining uh beyond compliance for december have a great one thank you guys thank you you
Show moreFrequently asked questions
What is needed for an electronic signature?
How do I add an electronic signature to my PDF using a Signature Field in airSlate SignNow?
How do I insert an electronic signature box into a PDF?
Get more for propose electronically signing Request with airSlate SignNow
- Official sign
- Prove electronically signed Building Quote Template
- Endorse digisign Car Receipt Template
- Authorize electronically sign Sales Proposal Template
- Anneal mark Parking Ticket
- Justify esign Medical Power of Attorney
- Try countersign Window Cleaning Proposal Template
- Add Joinder Agreement electronically signed
- Send Service Receipt Template byline
- Fax Recommendation Letter esigning
- Seal Artist Press Release signature block
- Password Website Standard Terms and Conditions Template signature service
- Pass Tolling Agreement countersign
- Renew Bank Loan Agreement signatory
- Test Go To Market Strategy initials
- Require Intellectual Property Assignment Agreement Template eSign
- Comment subscriber email signature
- Champion inheritor signature
- Call for visitor initial
- Void Terms of Use Agreement template digi-sign
- Adopt Nominee Agreement template esign
- Vouch Musical Ticket template signature block
- Establish Professional Birthday Party Invitation template signature
- Clear Construction Contract Template template email signature
- Complete Discount Voucher template signatory
- Force Offer Letter Template template digital signature
- Permit Landscape Transforming Appointment Record template electronically signed
- Customize Construction Contract Agreement template byline