Re-assign Countersign Request with airSlate SignNow
Improve your document workflow with airSlate SignNow
Agile eSignature workflows
Instant visibility into document status
Easy and fast integration set up
Re assign countersign request on any device
Detailed Audit Trail
Strict security requirements
See airSlate SignNow eSignatures in action
airSlate SignNow solutions for better efficiency
Our user reviews speak for themselves






Why choose airSlate SignNow
-
Free 7-day trial. Choose the plan you need and try it risk-free.
-
Honest pricing for full-featured plans. airSlate SignNow offers subscription plans with no overages or hidden fees at renewal.
-
Enterprise-grade security. airSlate SignNow helps you comply with global security standards.

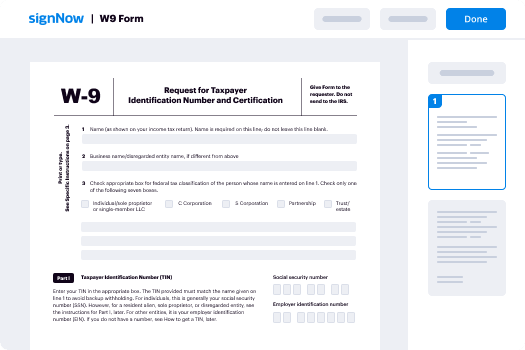
Your step-by-step guide — re assign countersign request
Employing airSlate SignNow’s electronic signature any business can speed up signature workflows and sign online in real-time, providing an improved experience to clients and staff members. re-assign countersign Request in a few simple actions. Our mobile-first apps make operating on the run possible, even while off the internet! eSign documents from any place worldwide and complete trades in less time.
Take a step-by-step instruction to re-assign countersign Request:
- Sign in to your airSlate SignNow profile.
- Find your document within your folders or import a new one.
- Open up the record and edit content using the Tools list.
- Place fillable fields, type text and sign it.
- Include numerous signers using their emails and set up the signing order.
- Specify which individuals can get an completed copy.
- Use Advanced Options to restrict access to the template and set up an expiration date.
- Press Save and Close when done.
Moreover, there are more innovative functions accessible to re-assign countersign Request. List users to your common digital workplace, browse teams, and keep track of cooperation. Millions of consumers across the US and Europe concur that a system that brings everything together in a single unified digital location, is what companies need to keep workflows working effortlessly. The airSlate SignNow REST API allows you to embed eSignatures into your app, internet site, CRM or cloud storage. Try out airSlate SignNow and enjoy quicker, easier and overall more productive eSignature workflows!
How it works
airSlate SignNow features that users love
See exceptional results re-assign countersign Request with airSlate SignNow
Get legally-binding signatures now!
FAQs
-
How do I re assign on airSlate SignNow?
From the envelope, click "OTHER ACTIONS." Click "Assign to Someone Else." Enter the new signer's email address, name, and reason for changing the signing responsibility. When finished, click "ASSIGN TO SOMEONE ELSE." -
How do I change my signature on airSlate SignNow without an account?
If you don't want a free airSlate SignNow account, you can ask the sender of the documents to correct the document without your middle initial as a part of your name and resend it to you. With your full name formatted differently, the airSlate SignNow system will prompt you to "adopt" a new signature. -
How do I correct my signature on airSlate SignNow?
From your airSlate SignNow Account, click your Profile image, then click My Preferences. Choose Signatures. Click Delete to remove an existing signature, or + Add New to create a new signature. -
Can you forward an airSlate SignNow?
We recently found out that if someone receives an airSlate SignNow email to sign a document, they can forward that email to someone else and that person who they forwarded it to can sign for them in the original recipient's spot and the original recipient's name will show up. -
How do I forward a document?
Open the file you want to send. In the Quick Access Toolbar, click Send to Mail Recipient to open an email message. Your file will appear in the body of the message. Enter the recipients' aliases, edit the subject line and message body as necessary, and then click Send. -
How do you write a countersign letter?
Understanding Countersignatures The first party reads the document and signs it if they agree to the terms of the agreement, the second party then countersigns the document by providing their signature confirming their agreement with the terms of the contract. -
Can your signature be anything?
Usually, a signature is simply someone's name written in a stylized fashion. However, that is not really necessary. ... The signature can be made by anything that marks the airSlate SignNow. Pencil is not favored because it can smudge and be erased, but a signature made with a pencil is equally valid as a signature in pen. -
What is a countersigned lease?
A countersignature is an additional signature that is placed on a document after it has already been signed. It is a way to provide authentication and confirmation. ... Most all contracts will have two signatures on them. The first party will read the agreement and sign if they are willing to take on the terms. -
How do you countersign a contract?
To countersign, head into the job or lead and under contract click the arrow down and then OPEN. This will open the contract in a sign-able tab where you can countersign it. -
What is a countersign contract?
Countersign (legal) From Wikipedia, the free encyclopedia. Countersigning means writing a second signature onto a document. For example, a contract or other official document signed by the representative of a company may be countersigned by his supervisor to verify the authority of the representative. -
How do you write a countersign application?
Suggested clip How to Countersign the Application Form and Photo - YouTubeYouTubeStart of suggested clipEnd of suggested clip How to Countersign the Application Form and Photo - YouTube -
Who can countersign a UK passport?
Who can sign your form and photo. Your countersignatory must: have known you (or the adult who signed the form if the passport is for a child under 16) for at least 2 years. be able to identify you, for example they're a friend, neighbour or colleague (not just someone who knows you professionally) -
What is TC countersign?
Counter sign on TC / School leaving certificate shall however be submitted to the school positively at the time of admission. And In case, someone is migrating from different state within India, the Transfer Certificate (TC) duly countersigned by the CBSE Regional Office. -
How do you alter a contract?
There is no specific time for changing the whole or part of your contract. As long as both parties are in agreement, the process can take off. In the case of minor modifications, the parties can handwrite them and include them in the original document. They can sign or include after handwriting the changes. -
How do you do a countersignature?
Suggested clip How to Countersign the Application Form and Photo - YouTubeYouTubeStart of suggested clipEnd of suggested clip How to Countersign the Application Form and Photo - YouTube
What active users are saying — re assign countersign request
Re assign countersign request
thank you very much all right like I have control here so I want to start before we dig into the presentation itself and just spent one minute to thank the companies we've really seen a lot of people and a lot of companies stepping up and helping out during that the current coated crisis and seeing some some great deeds by the applicants themselves and so I really want to call attention to that and thank the companies who have been extremely responsive and and really in many cases stepping up and looking in the best interest of the public during this time and at the same time too I want to thank the folks on the aMDA program team because we've seen some real heroic work on sort of this side of Defense too and that's the folks in the office of generic drugs in the office of pharmaceutical quality and of course there's a lot of other people supporting the program across the center but especially in these two groups we've seen them really understand the importance and jump on the applications I've seen supplements that were approved in a matter of a couple days I saw one worthy acknowledgement letter was issued one day and then the approval action was issued the next day and so I really appreciate the fact that everybody is working very hard so with that I'll sort of focus back now to the regular program here and we're going to talk a little bit today about the aMDA program performance and go through some of what the data means and then and where we can provide some tips to help you sort of navigate this process okay we're gonna walk through some of the key program metrics we'll provide some tips for success and then we'll also look at some of the resources that people can use to help better navigate the process so the agenda will start pre AMD a program right this is the in subject of the whole thing we will move forward and cover the entire process from looking at filing to review to approval to post approval submissions starts with the product specific guidances so one of the big successes of the GU duper program is the research program and its ability to focus on efforts that directly benefits the review process one of the most visual outputs of that program is the product specific guidances these provide great insight into the bioequivalence thoughts around the product it's also facilitates sort of our ability to bring the program especially the bioequivalence program is adequate first cycle around seventy percent or so of the time that's an enormous win for both of us right the company knows what the target is reviewers know what the target is we'll certainly take other recommendations but people can see what our expectations are and we're able to use this to really drive the Bible problems process specifically to that first cycle adequate that's one of the key components and our ability to move these applications through the process much faster and we'll show you a little bit of that later on we also have had the team sort of break off their efforts into looking at both non-complex right the more traditional products and then a big push to get better direction out there on the complex 5x products that a little trickier to work on so these efforts are continuing take a look at those it's great insight into what's our thinking or if you have suggestions certainly other paths to bring that information forward to the agency one of these is the pre a a meeting request program so this is something that was formalized under we do so to working very successfully it's a great way to sort of sound out your path forward for these more complex products and interact with the team so that we all have a better understanding of what the application is going to look like and where some of the key points may be cold correspondence is another opportunity for potential applicants to gain some clarification on the path forward and what we've seen here is a steady increase in companies using this path right very beneficial tool there are some of the reasons that we've had to reject controlled correspondence I'm not going to go through all these but the key is the more effort you put into your controlled correspondence the more value you will receive out of the program and sort of show you what's going on sort of matching the submission rate we've been taking the control correspondence very seriously big push to make sure we're able to answer these controls give you meaningful answers and try to do it very quickly in sort of corresponding to the record levels of submissions we've had record level of output and I really want to thank the people who worked on control correspondence 29 18 and 19 to allow us to set the record last year so big win for both of us also want to look at then some tips for control correspondence like please make it easy for us to understand your request right and one of the keys to doing that is to make sure that your cover letter clearly indicates whether you have attachment the number of attachments and provide the appropriate background information like help to clarify exactly what you're looking for in certainly locking in things such as the reference list the drug the RLB even the dosage form can help us give you a better answer I can that's to both our advantages there are some resources to help better navigate the controlled correspondence process and some of the other sort of pre aMDA program efforts better going on and we're going to pivot and look at the filing and by this we'll look at the a.m. day receipts as you can see the receipts fluctuate significantly from year to year we also see fluctuations from month and even within the month with trends being that much of these submissions occur within the last few days of the month it certainly presents some operational challenges but the team has been very diligent and working forward and moving those applications through and I'm going to look at the refuse to file percentage this is something that has changed in recent years I think this is a big benefit to the program right we're having more communications and interactions during the filing period right there were updates to the guidance that helps facilitate these interactions the filing team's assessment and its checklist it's public but you can see the exact same sort of assessment tool that we are using to go through these applications to make sure they contain all of the right pieces in applicants I think are using this checklist to build a better application so we certainly appreciate that we are seeing a higher quality submission coming through and refusing it's not what we want to do and what you'll see here is sort of the impact of the applications and so there's certain been a little bit of attention to the drop and the number of submissions but it's not as significant as people think because the key what's being worked on to the disciplines that number is actually a little bit more stable right because we refuse fewer applications it means applications are coming in and landing what's the team write that means that we're able to start the assessment process like moot those applications hopefully down the path towards approval talk a little bit impact of refusing applications like this is not the desired state right we don't want to refuse applications that's not helping us meet our mission right however there's a lot of pressure for us especially in the NDA program to follow the procedures to be consistent sometimes unfortunate but we have to treat everybody as consistently as possible to be in a position to sort of defend actions that we take and defend the robustness of the systems that we all cooperate within so I want to go through a couple other points and really highlight this like there's nothing in it for FDA to refuse an application we don't want to refuse applications it's actually more work for us to refuse to applications right we've got a double check everything we have to have more meetings we have to alert people that we're going to refuse an application oftentimes these results in disputes that's a significant amount of effort on errand and most importantly for both of us but it can delay our ability to bring an application towards approval and that's what we all want right we need those approvals and we especially need those approvals for critical products our mission is to bring high quality Jerr drugs to the market we can't do that if we can't both work together to get them approved or provide some Island tips and I'm not going to go through each one of these but the key is do your homework it will help us both manage these applications now we're going to shift gears and look at the actual submission and invest in a quality application we can't say that enough do it do it right this will help us both it saves you time it saves money in terms of filing fees the late market entry and one of the to help us sort of better navigate your submission is to make sure the cover letter is clear and it tells us what's in the submission knowing what's in the submission allows us to assign the submission to the correct team members hidden information is a common reason for stress within the team miss goal dates and approval delays great so help us mitigate these circumstances and so here we'll provide some sort of tips specifically on the Commission when you're referencing we called the pre submission facility correspondents please clearly submit that and make sure it ties in directly to the actual application right we want to keep these two documents in alignment the same set of facilities in the PFC should be matched in the a and da itself that's going to allow us to move these packages forward and reduce the time to approval note in the PFC then the subsequent aMDA the priority request if it's not a priority application that you intend to submit then you shouldn't be using the TFC process at the same time when we submit that TFC make sure you justify the priority request and then repeat that fancy prime da MVA submitted these will go a long way to help facilitate us processing the application and also please note if that application is part of the pre-mba program as you already know there's some extra sort of care and heating that goes on to those companies and so if we can figure that out up front we can start to arrange mid-cycle meetings and those kind of opportunities for the companies that have the appropriate submission now another thing to think about is who's going to be doing the review we call that the Assessor what are they looking for and let's help them make sure they get that and so do you want your Assessor searching for the data or you want them evaluating the data consider what we're going to be looking for and provide that build a reputation for quality submissions right make it easy for us to navigate the application they can convey the appropriate message show us you have things under control and can submit everything and when you're looking at the actual data remember the Assessors they love to analyze data that's what it's all about for them so give it to them make sure it's completely presented don't have any gaps use clear labels and figures make it easy to read and compare make sure your data supports the overall story of the application like we're submitting this drug when you have confidence in it here's why we are confident made sure that message gets carried over to the team that's making the assessment right link it in so what's that you know your product if you're referencing prior communications with the agency make sure you fully cite any suitability or citizen petitions use the appropriate docket number right that will help us both be looking at the same information at the same time and using that to sort of help your application move forward if you're studying a controlled correspondence again make sure you have the original request and our response built into the application and then again if you've had meetings with the agency like reference notes you make that very clean and show us the value for today don't reference something that doesn't have value or where you have changed sort of the base of your application after meetings or after control correspondence right make it as easy as possible for us to understand you have things under control if you need to deviate from a guidance perfectly acceptable right there's a lot of value for us to understanding how you're thinking by showing us these deviations but clearly note that you're deviating they make it rational here is why I think this alternate approach makes sense they explain through data all right think about your audience again they're gonna want to look at the data you're making a proposal back it up with the numbers they what you do you what's your say prove it improve it right show us that in the application show us that you know your product capture that knowledge meet the criteria right oftentimes we're stuck you haven't met all regulatory criteria we're forced to give you a complete response letter comply with any requests you get from the team hey completely address the deficiencies like don't race to get it in do it right and then repeat all of this for your contractors it's very important to understand contractors have an equal role in your success through this process so you use responsible contractors especially with the drug master file some of these other key aspects of your product communicate with us right make sure they know your key dates you know what they're submitting when there's so many FDA shouldn't be the link between these two parties and make sure you know and list all the facilities that are going to be used and here's sort of a staggering statistic from our friends in the office of pharmaceutical quality 25% of the DMF that companies are relying on that hidden facilities that has enormous consequences for our ability to move forward on an application and minimum it's going to trigger another amendment coming in that's going to impact the goal base so here's some additional resources to help sort of navigate through that process now we're going to pivot and look at the mid-cycle communications right this is an opportunity then within the middle of the review cycle for us to communicate some thoughts that we have about your application provide a little insight into some of the areas that we're focusing on some of the areas where they may be concerned or simply requesting clarification or additional information to sort of help us move through our assessment so what you see here is the volume of communication so this is amazing thousands of these mid-cycle communications going on each year this it's been a big success as part of the Dubuque program it's one of the key pieces to us reducing the number of cycles it takes to approval and then subsequently the total time it takes to approve an application there are some tips to help better navigate the mid cycle process first order from your deficiencies see if we can minimize it right are there less employment one application that you can apply to the next application provide complete and timely responses right we're gonna give you a response time try to meet that if you can't meet that contact the appropriate people the goal dates are very important for us right and we're constantly sort of monitoring what are we getting from the applicant when are we getting it how does that fit with our scheme for moving this application forward within that goal cycle if you experience any delays on your end talk to the discipline project manager Thanks that's going to be the key contact for you on this if you don't respond and this happens it happens more than we expected where people aren't able to respond to the IR or DRL that forces less then to issue the formal complete response letter like extra resources on our end to make this happen and then we want to pivot a little bit because a small set of applications and as we saw earlier growing set of applications are eligible not just for getting the mid-cycle communications in terms of the information requests and discipline review letters but there's an additional communication piece built into the canoe for program where we will schedule a mid cycle meeting with you right and so this will be scheduled by the regulatory project manager early in the cycle don't start to reach out contact applicants scheduled this meeting at a future date and so as you start to get information requests and discipline review letters you can send rpm sort of your timeline and when you think you'll be able to respond to this another thing that you can do is send questions right there's no obligation for us to respond to those questions but we will make a good-faith effort if you give us a reasonable question shows you've done the homework where you provided some of the appropriate background not just where do I go FDA but here's what I'm hearing here's what I'm seeing here's what I think you want does this sort of help you to better understand my application then we will make a good-faith effort to try to address that during the meeting the key though is giving us plenty of warning about those types of communications so again there's a link for some more information about that sort of facet of the program now we'll focus on the end cycle communications what you see here are the complete response letters right now a little bit of history even before the complete response letters we're sort of fully implemented through to do for one and to see again like several other aspects of the program big increase in the amount of communications and this shows you how the applications are flowing through the program like this is happening at a much better pace than it was historically the complete response tips alright let's take advantage of this again learn from your deficiencies all right a lot of overlap with the early communications don't provide extraneous information if we ask you a simple question you know can you provide data point X give us that give us X don't give us the entire alphabet if you send more in we're obligated to look at it right and that looks may cause us to ask other questions right and so give the reviewer exactly what he or she is looking for be accurate be consistent write your response should tie in to that bigger story of the application that we're both working through be timely right help maintain our momentum but people sort of don't understand how the teams or team works on an application when they have a formal response that goes out like that team now has to grab a new application and start working on so the quicker you can respond the more we can maintain the momentum the energy and it's much easier and better for the team get those responses get them back into play while it's fresh with the reviewers these tips hopefully close out your next cycle with an approval that's what we all want and then we'll expand a little bit because we want to highlight a couple other aspects that people can sort of utilize better to help the flow of their application first be very focused on the timing of your changes right then unsolicited changes from the applicant or even from your drug master file holder have significant consequences to our ability to move forward with the application he diligent watch for the external changes right the reference listed drug could make labeling changes United States pharmacopoeia could be updated those are type of changes you need to be on top of submit updates to your application as soon as those changes are made don't wait for the invitation from the program to do that jump on this like the factor you make that change the better it is for you we can't say it any easier than that people oftentimes wait for us don't wait send it in and then if you have a tentative approval and want that to be converted to a full approval send those in three months prior to that anticipated full approval date there's some resources here and really the key instead of providing a bunch of links if you need to navigate your application the best resource is Rachel a project manager right you can reach the project manager right they're off on other activities we post the team leaders and the supervisors all that's available you should have no trouble sort of working through the Katori project management structure these are the people who know best about what's happening with your application and what the impact would be for changes and things of that nature so use them right that's their job they are your champion for your application and they are dedicated to moving these applications forward so I won't go through all of these different points but here's where there's more value in talking to the project manager like there's different time frames where it does make sense to reach out to the project manager and in many of these cases the project manager will proactive in reaching out to you and hey hey it looks like we're going to have a missed date here's what's going on here's when we anticipate being able to provide sort of the response at the same time there a couple cases whereas very limited value to reaching out to the project manager right oftentimes we get people asking for the status of their application a couple weeks after it was received right in normal circumstances we're not going to have that information right away right and so the conscientious and respectful of the project managers right and try to use them when it's appropriate they will work with you they will be your advocates within the process then of course write the approval this is what everybody is racing to get right we want these you want these we can see is the program willing has taken the ability to process the applications to that next level the approval numbers are certainly a great reflection of the success that we have and you know it's great we built a program we can approve lots of applications we're at a very healthy point in our ability to process application so you give us something that's reasonable we will work with you we will get it approved and we're also seeing now is not just the ability to get approvals out the door but to do it faster right and this certainly helps the companies get a better understanding in terms of what the expected time to approval will be right get you a little bit more business certainty we're taking our commitments very seriously we knew we had to do a better job managing the application and so what you see here is really significant significant improvement over the time it takes to get sort of comparable numbers of applications or proofing and certainly there's outliers special is here and there but if you're submitting a typical type of product and you've done it right this is the type of treatment in a type of timing that you can expect to get from the program supplements like the story of the application doesn't end with the approval we're gonna continue to work on this applications like this starts an entire new sort of chapter in our regulation of the product and so again we're gonna have to come together to work successfully through this process and what you can see is a big increase in the number of submissions and this is really due to two factors and of course immediately people are going to say well there's no C for the prior approval supplements under get it for two and that's why people are so many more that's certainly part of it but one of the things we've seen with record numbers of approvals we're now generating record numbers of supplements and we certainly I think all understand that the application that's newly approved the first a year or so we tend to see a higher number of supplements it's not that supplements ever stopped from applicants but I think we all know there are a little bit more intense shortly after they're approved that's fine that's understandable and this makes sense with more approvals we're generating more prior approval supplements there's also something that we need to understand when we're looking at the entire aMDA program not just originals we must also factor in the growing number of post approval activities that are going on and so what you see here is a combination of both the prior approval supplements and the changes being affected supplements this number is gone up and up it's enormous right closing in at 9,000 supplements last year think about that divide that by sort of the number of staff members that we have and that becomes a staggering amount of work so when we look at the supplements again we're trying to to match the pace that's coming in we just can't do that right it's enormous growth over the past few years right from the receipt size we're trying to get as many out the door as we can but certainly we're not able to match that growing trend we don't have the has to be to match those be respectful when you submit your changes in we will seriously we will work on them but at the same time I don't think anybody envisioned the volume that we're sitting here today and so we are as you can see from this slide losing ground now certainly a number that we didn't show here with the complete responses that go out I don't want to show that we want these to be approved right and so we understand the supplements are very important to you give us the data use those same tips we talked about right read your audience give us the appropriate data demonstrate you know what's going on in the supplement you can tie those supplement changes back to the original application right that becomes part of your story on the application right here's what we're doing here's why we're doing it here's why it makes sense and here's the proof that's what we're looking for in this case then we'll shift and look at some tips first like we said the story doesn't end there right continue this is essential we are going to continue to work with you for the life of that product an awesome very important the quality of your submissions doesn't end with the approval maintain that same high standard of quality that's the key to us moving forward and allowing you to make those changes right and it carefully assess the nature of the change before determining which type of reporting mechanism is appropriate to give us a change that we think makes sense but you submitted through the wrong classification that slows us down it may be forced to resubmit that the appropriate way right that's going to delay your ability to implement that change and so take that just as serious as you took the original application and then the court make the links right as part of telling the story you've got to be able to link in here's why this change makes sense here's the part of the application that we're changing here's the reference here's why we can make this change successful and maintain sort of the high profile right in the high quality of this product through this change and of course we take these goals very seriously right there's a lot of iearned right there's a lot of reporting built in to the bt-50 program right we're proud where we are we know when our persitz were continuing to fine-tune and improve the program and we will give you reports like we're happy to show what's going on and we want there to be transparency so you see a wide range of reports from monthly reports from some of the key statistics a little bit expanded for the quarterly reports and then of course there's an annual report for Congress where you'll see this data any more data points about the program and we also know if we can't meet the goals we're gonna have to explain the misses right and so we have to explain to Congress government accountability office right Gao if we miss they're expected to audit and they're expected to audit even when we make the goals right they come in periodically and audit us and so we know that's coming we want to do a good job we want to be able to focus our staff on the applications and not be distracted with some of these other activities so if we're successful and you help us be successful we can dedicate more and more of our efforts to moving the applications forward and not have sort of the sidebar distractions occurring so want to go through a couple challenge questions right does FDA want to refuse to receive an application they say yes I hope not no way we don't want this we need the applications to come in that's our lifeblood we can't help the public by making generic drugs available if we don't get the application and we can't file those applications so we put a lot of effort into trying to make that program run as successfully as possible write the story of your application does not end with the approval right hopefully people will walk away from that continue write that same effort that same diligence on your application bring that all the way through a lot of times we see people try very hard to get the application approved then their post approval changes or sloppy right they've turned it over to different people they can't link back to the original application know your application know your story be able to communicate that story to our post approval team right and we're seeing more and more of these changes coming in so it's even more important that you can help us manage this growing workload by giving us the right information and providing the appropriate references data links and connections back into the original submission to help us better navigate so with that have some concluding remarks here first day and day programs in great shape we've shown that we can process high volume applications we've shown that if you give us quality applications we can process them and bring them to approval very quickly right seeing the fastest approval times and the Holga doofah era going on right now right we're on it we know what's going on we know how to move the good applications forward we're trying to help people understand the tips to build a better application that allows us to move them forward that allows you to get that approval forward and most important to allowing the public to get the benefit of those applications right pre submission communications those are increasing take advantage of this like that is a wonderful program if you're working on one of these novel products more complex type of products take advantage of that if you have something different could be a more traditional product but you want to do something a little different again use this program right there's an opportunity to get information from us but at the same time make sure you're doing your homework don't ask general questions here's what we're thinking here's why we're thinking here's why we think this is supportable like that's the type of pre-submission communication that's going to allow us to have a much better interaction and allow us to help you get that application pulled together to be successful then you're in a position a better chance of being accepted right we've had some sort of expanded communications that help to success right here grow we want that to increase right we want to file these applications we need them filed take advantage straight do it right work with us we'll get them filed take advantage of the within cycle communications right we're seeing more and more communications especially in that first cycle that we're seeing multiple rounds of communications from many of the disciplines take advantage of that right give the team what they need and that's going to help us get more first cycle approvals right and that's the ultimate goal would be that pivot easily you've seen that we're exceeding how we could do for two goals right we want that to work we want it to be successful we're putting pieces in place to make it successful everybody knows what's expected of them like reviewers the project managers the management team were all pulling together to make this work and the key to if it's people from all over the program taking office that's supporting the a MBA program they're on it everybody knows what's going on we're all reading from the same script we're all linked together to make this successful and so I think we certainly seen more of a comprehensive team effort in the more recent years than ever was within the program and for somebody like me who's been in a program for a long time it's wonderful now to say hey we've got a question over here and we don't even have to explain to some of the other people around the agency who we are but I can member call many people years ago hey I'm working on this generic application right we're stuck I can't move forward with my aMDA until you answer this question and you would get that timeout moment what's an AMTA who are you where are you coming from right we don't have that now when we say we're working on an AMD a right people listen they're responsive they run us to be successful they're part of our team right there's not a we in a day we're all part of this larger a NBA team and so that has been working very successful and having the user fee program I think has really tied us all together to be on the same page right the assessment time with us is dropping right but goals matter I know they matter to you they matter to us too and nothing helps sort of generate people working and think than having those gold dates in place right we need them you need them for business certainty that's working that's successful right we've done it's a struggle there's a lot of sweat and effort to get the application done on time but we're doing that right it's a very brisk pace for us to get these applications done but it's happening and it's happening successfully and of course with that success the approval times are dropping like that's wonderful that's a great metric it's certainly one of many of the tricks but we know this is one that gets a lot of attention that's great right it's also happening better on the post approval side right all of these shows that it is a great time to submit in application into the generic program taking them very seriously on it and things are happening right and we appreciate all of the efforts and support and at this point Jeff will sort of pause here
Show moreFrequently asked questions
What is needed for an electronic signature?
How can I use my phone to sign a PDF?
How can I include an electronic signature in a Word document?
Get more for re-assign countersign Request with airSlate SignNow
- Print electronically sign Congratulation Letter for Promotion
- Prove electronically signed Term Sheet Template
- Endorse digisign Termination Letter Template
- Authorize electronically sign 1040 Form
- Anneal mark Summer Camp Certificate
- Justify esign Home Remodeling Contract
- Try countersign Building Quote Template
- Add Severance Agreement electronic signature
- Send Restaurant Receipt Template signed electronically
- Fax Promotion Cover Letter electronically sign
- Seal Book Press Release electronically signing
- Password Project Management Proposal Template mark
- Pass Toll Manufacturing Agreement signed
- Renew Money Loan Contract autograph
- Test Lean Business Model Canvas digital sign
- Require Letter of Intent Template initial
- Comment cosigner eSignature
- Champion receiver eSign
- Call for renter initials
- Void Affidavit of Identity template countersign
- Adopt Split Dollar Agreement template sign
- Vouch Parking Ticket template electronically signing
- Establish Character Profile template eSign
- Clear Solar Panel Installation Proposal Template template eSignature
- Complete Website Design Request template autograph
- Force Memorandum of Understanding Template template digisign
- Permit Landlord Verification Form template electronic signature
- Customize Firearm Bill of Sale template signed electronically